Introduction
Enantiornithes is the most speciose clade of Cretaceous birds, with >50 genera named to date (Zhou, Reference Zhou2014; O'Connor et al., Reference O'Connor, Wang, Zheng, Hu, Zhang and Zhou2016; Chiappe et al., Reference Chiappe, Meng, Serrano, Sigurdsen, Wang, Bell and Liu2019; Wang and Zhou, Reference Wang and Zhou2019; Xu et al., Reference Xu, Zhou, Wang and Wang2020). Fossils belonging to this group have been reported from every continent except Antarctica (Chiappe and Meng, Reference Chiappe and Meng2016). The Early Cretaceous Jehol Biota in northeastern China has produced more than half of the known enantiornithine birds (Hu et al., Reference Hu, O'Connor and Zhou2015, Reference Hu, O'Connor, Wang, Wroe and McDonald2020; Wang and O'Connor, Reference Wang and O'Connor2017; Wang and Zhou, Reference Wang and Zhou2019). Most specimens from the Jehol Biota are nearly complete and often preserve plumage or other soft tissue remains. The abundant fossil birds from the Jehol Biota not only have increased the taxonomic diversity of the Cretaceous birds, but also have provided critical information regarding aspects of the ontogeny, sexual dimorphism, reproduction, and ecology (Zhang and Zhou, Reference Zhang and Zhou2000; Zhou and Zhang, Reference Zhou and Zhang2006; O'Connor et al., Reference O'Connor, Wang, Chiappe, Gao, Meng, Cheng and Liu2009; Zheng et al., Reference Zheng, Martin, Zhou, Burnham, Zhang and Miao2011, Reference Zheng, O'Connor, Wang, Zhang and Wang2014; Wang and Zhou, Reference Wang and Zhou2019; Bailleul et al., Reference Bailleul, O'Connor, Li, Wu, Zhao, Martinez, Wang and Zheng2020). With increasing information provided by continuous discoveries of enantiornithines, the analysis and interpretation of their evolutionary history, interrelationships, and ecology have been proved challenging tasks (Wang and O'Connor, Reference Wang and O'Connor2017; Chiappe et al., Reference Chiappe, Meng, Serrano, Sigurdsen, Wang, Bell and Liu2019; O'Connor et al., Reference O'Connor, Zheng, Pan, Wang, Wang, Zhang and Zhou2020). The majority of the Jehol Biota enantiornithines have been referred to four main groups: the small-bodied and relatively unspecialized cathayornithids (sensu Wang and Liu, Reference Wang and Liu2016; see also O'Connor and Dyke, Reference O'Connor, Dyke, Boles and Worthy2010), the long-snouted longipterygids (O'Connor et al., Reference O'Connor, Wang, Chiappe, Gao, Meng, Cheng and Liu2009), and two groups of large-bodied taxa—the bohaiornithids (M. Wang et al., Reference Wang, O'Connor, Zheng, Wang, Hu and Zhou2014) and the pengornithids (X.-L. Wang et al., Reference Wang, O'Connor, Zheng, Wang, Hu and Zhou2014). The monophyly of some of these groups is debated, in particular for the cathayornithids and the bohaiornithids, because both might represent evolutionary grades among Euenantiornithes (see Wang and Liu, Reference Wang and Liu2016; Chiappe et al., Reference Chiappe, Meng, Serrano, Sigurdsen, Wang, Bell and Liu2019).
Here, we describe a new enantiornithine bird from the Lower Cretaceous Jiufotang Formation in western Liaoning of China. The new enantiornithine combines size and peculiar features previously seen in small-bodied enantiornithines and in the cathayornithids with features usually identified as diagnostic of the bohaiornithid lineage.
Material and methods
The new specimen (MHGU-3000) described here was collected from the Lower Cretaceous Jiufotang Formation (Aptian) at Shangheshou locality, Chaoyang City of western Liaoning, China (41°36′N, 120°23′E) (Fig. 1). In order to determine the ontogenetic age of MHGU-3000, we took a sample from the middle shaft of the left tibiotarsus of the type specimen. The sample was embedded in one-component resin (EXAKT Technovit 7200) and hardened in a light polymerization device (EXAKT 520). The histological section was cut transversely using an accurate circular saw (EXAKT 300CP). The section was ground using the EXAKT 400CS grinding system until the desired optical contrast was obtained. The histological section was examined by light microscopy under both normal and polarized light (ZEISS AX10). Images of each slice were captured using a digital camera (ZEISS AxioCam MRc5) and further processed using Adobe Photoshop and CorelDRAW X5 software. Histological measurements were taken from digitized cross sections using ImageJ.

Figure 1. Map of Liaoning Province, China, showing the Shangheshou locality in Chaoyang City where the holotype (MHGU-3000) of Musivavis amabilis n. gen. n. sp. was recovered.
In order to place MHGU-3000 in a phylogenetic context, we coded it as an Operational Taxonomic Unit (OTU), and included into a data set expanded from the phylogenetic analysis of X.-R. Wang et al. (Reference Wang, Shen, Liu, Gao, Cheng and Zhang2020; modified from Cau, Reference Cau2018) focusing on Mesozoic short-tailed birds. The matrix consists of 86 OTUs scored for 1837 binary character statements (Supplementary data 1, 2). The long-tailed bird Archaeopteryx was used as outgroup to root the tree. All analyses performed 1000 ‘New Technology search’ replications using the TNT software (Goloboff et al., Reference Goloboff, Farris and Nixon2008) in order to explore tree islands, and then explored more exhaustively running a Tree-Bisection-Reconnection analysis using the sampled trees as starting point. We explored the effect of homoplastic characters on pygostylian relationships performing four analyses using the “Extended Implied Weighting” function in TNT, setting the concavity parameter K value (which defines the downweighting of the characters according to their homoplasy) as, alternatively, 5 (most aggressive downweighting setting used), 10, 15, and 20 (less aggressive downweighting setting used). The four replications using the “Extended Implied Weighting” function followed the same search protocol used in the first (unweighted) analysis.
Following O'Connor and Dyke (Reference O'Connor, Dyke, Boles and Worthy2010), we consider Vescornis a junior synonym of Hebeiornis. The clade name Bohaiornithidae refers to the least inclusive clade containing Bohaiornis and Shenqiornis (M. Wang et al., Reference Wang, Zhou, O'Connor and Zelenkov2014), whereas the informal terms “bohaiornithid-like” and “bohaiornithid-grade” refer to bohaiornithids and to those enantiornithines sharing a subset of the diagnostic features of Bohaiornithidae regardless to their inclusion in the latter clade (e.g., Gretcheniao, Chiappe et al., Reference Chiappe, Meng, Serrano, Sigurdsen, Wang, Bell and Liu2019).
Repositories and institutional abbreviations
Types and other specimens examined in this study are deposited in the following institutions: The Museum of Hebei GEO University (MHGU), Shijiazhuang City, Hebei Province, China; Beijing Museum of Natural History (BMNH), Beijing, China; or the Institute of Vertebrate Paleontology and Paleoanthropology (IVPP), Beijing, China.
Systematic paleontology
Avialae Gauthier, Reference Gauthier and Padian1986
Pygostylia Chiappe, Reference Chiappe, Chiappe and Witmer2002
Ornithothoraces Chiappe, Reference Chiappe1995
Enantiornithes Walker, Reference Walker1981
Genus Musivavis new genus
Type species
Musivavis amabilis n. gen. n. sp., only known species, from the Aptian of northeastern China.
Diagnosis
As for the type species, by monotypy.
Etymology
The generic name is derived from the Latin “musivum” (mosaic, decoration with small stone tesserae) and “avis” (bird).
Holotype and only known specimen
MHGU-3000, a nearly complete and articulated skeleton preserved in a single slab.

Figure 2. Holotype (MHGU-3000) of Musivavis amabilis n. gen. n. sp. from the Early Cretaceous Jiufotang Formation (Aptian). (1) photograph; (2) line drawing. Abbreviations: al, alular metacarpal; ald, alular digits; an, angular; cav, caudal vertebrae; cv, cervical vertebrae; d, dentary; fu, furcula; fr, frontal; hy, hyoid; il, ilium; lco, left coracoid; lfe, left femur; lfi, left fibula; lh, left humerus; lr, left radius; lsc, left scapula; lti, left tibiotarsus; ltm, left tarsometatarsus; lu, left ulna; m, maxilla; m-Ⅰ–Ⅳ, metatarsal Ⅰ-Ⅳ; mac, major metacarpal; mad, major manual digits; mic, minor metacarpal; mid, minor manual digits; n, nasal; pa, parietal; pd-Ⅰ–Ⅳ, pedal digit Ⅰ-Ⅳ; pr, premaxilla; pu, pubis; py, pygostyle; rco, right coracoid; rfe, right femur; rfi, right fibula; rh, right humerus; rl, radiale; rr, right radius; rsc, right scapula; rti, right tibiotarsus; ru, right ulna; se, semilunate carpal; sr, scleral ring; st, sternum; su, surangular; syn, synsacrum; ul, ulnare.
Table 1. Measurements (in mm) of the holotype (MHGU-3000) of Musivavis amabilis n. gen. n. sp. Abbreviations: L, left; R, right; # indicates preserved length.

Diagnosis
Musivavis amabilis n. gen. n. sp. can be referred to Enantiornithes by possessing several synapomorphies of this clade, such as the sternum with nearly equal length and width, the “Y”-shaped furcula with a long hypocleideum, the minor metacarpal extending farther distally than the major metacarpal, and the fourth metatarsal relatively thin (Chiappe and Walker, Reference Chiappe, Walker, Chiappe and Witmer2002). It can be further referred to the “Bohaiornithidae-grade” group by possessing some of the diagnostic features of this family, such as the subconical teeth with tapered and slightly caudally recurved tips, the sternum with lateral trabecula projected caudolaterally, the omal end of furcular ramus with blunt expansion, and the tapered pygostyle without abrupt distal constriction (M. Wang et al., Reference Wang, O'Connor, Zheng, Wang, Hu and Zhou2014). It is distinguishable from the bohaiornithids by possessing the following unique combination of features (local autapomorphies denoted by asterisks): the pygostyle abruptly tapers distally (shared with Gretcheniao); the sulcus excavating the epicleideal ramus of the furcula is very deep and extends distally onto the proximal third of the hypocleideum*; a sharp keel runs along the whole ventral surface of the furcula, from the junction of the epicleideal rami to the distal tip of the hypocleideum; the flat cranial margin of the sternum becomes curved near the craniolateral process*; the sternum possesses craniolaterally projected craniolateral processes* (in bohaiornithids, only Zhouornis possesses craniolateral processes, which project laterally); the xiphoid process of the sternum extends nearly to the same level of the distal end of the lateral trabecula and expands as a blunt distal end (shared with Bohaiornis); and the first alular phalanx bears a transversally expanded proximal end followed by a gracile shaft, resulting in a concave proximomedial corner of the bone*.
Occurrence
The holotype was recovered from the Lower Cretaceous Jiufotang Formation (Aptian) at Shangheshou locality, Chaoyang City, Liaoning Province, China.
Description
The skull is mainly preserved in ventrolateral view (Fig. 3). The right premaxilla is preserved in lateral view. It gradually tapers rostrally and slightly expands dorsoventrally in the middle part, differing from the robust premaxilla of the bohaiornithids (M. Wang et al., Reference Wang, O'Connor, Zheng, Wang, Hu and Zhou2014) and the elongated premaxilla of the longipterygids (O'Connor and Chiappe, Reference O'Connor and Chiappe2011), but similar in overall proportions to those of the Cathayornithidae (Wang and Liu, Reference Wang and Liu2016). The frontal process of the right premaxilla is elongate and projects caudodorsally, but does not extend to the orbit, comparable with the condition in bohaiornithids (M. Wang et al., Reference Wang, O'Connor, Zheng, Wang, Hu and Zhou2014; Hu et al., Reference Hu, O'Connor, Wang, Wroe and McDonald2020). As in other bohaiornithid-like taxa, the maxilla is robust, with a straight ventral margin and gently expanded dorsal margin. The nasal is too broken to determine the exact anatomical features. The right lacrimal is preserved in dorsolateral view and appears to be “T”-shaped. The rostral ramus is oriented rostroventrally, but the exact length cannot be determined because it is overlapped by the right maxilla. The caudal ramus projects caudodorsally and the ventral ramus extends caudoventrally. The dorsal margin is concave at the middle part, as reported in Pengornis, Longusunguis, and Parabohaiornis, but different from the straight margin of Pterygornis (M. Wang et al., Reference Wang, Hu and Li2016). The frontal is rostrocaudally elongate, narrow rostrally, and domed caudally, as in other enantiornithines (e.g., Protopteryx, Parapengornis, Bohaiornis, and Shangyang; Zhang and Zhou, Reference Zhang and Zhou2000; M. Wang et al., Reference Wang, O'Connor, Zheng, Wang, Hu and Zhou2014; Hu et al., Reference Hu, O'Connor and Zhou2015; Wang and Zhou, Reference Wang and Zhou2019). The rostral portion of the frontal is rectangular-shaped, with a triangular depression, a feature that is also present in the enantiornithine Piscivorenantiornis inusitatus (Wang and Zhou, Reference Wang and Zhou2020) and the ornithuromorph Archaeorhynchus spathula (Zhou et al., Reference Zhou, Zhou and O'Connor2013). A deep oval fossa is located between the forked rostral end and the expanded dome of the frontal. This fossa, which is natural judging by the smooth margins and surface, has not been documented previously in Mesozoic birds. However, it is premature to conclude this oval fossa is a diagnostic character because the skulls of fossil birds are typically incompletely preserved or exposed in views that prevented observing this region. It probably represents the depression of the olfactory bulb and the articulation with the mesethmoid (Baumel et al., Reference Baumel, King, Breazile, Evans and Vanden Berge1993). The ventral margin of the frontal is slightly concave and forms the dorsal margin of the orbit. The dorsal margin of the frontal is robust and strongly expanded dorsally. The sclerotic ring is nearly completely preserved and, as preserved, is composed of 14 thin imbricated elements. The ventral seven sclerotic ossicles are much larger than the dorsal seven ossicles. The square-shaped sclerotic ossicles are plate-like and have rounded margins, similar to those of Longusunguis (Hu et al., Reference Hu, O'Connor, Wang, Wroe and McDonald2020). The quadrate is partly exposed in lateral view. No orbital process can be observed due to the overlapping by other bones. The rostral portions of the two strap-like jugals are exposed in lateral view, and disarticulated from the jugal processes of the maxillae. The right parietal is displaced above the skull and close to three cervical vertebrae. This condition proves that the parietal is not fused with the frontal. The palatal elements are mostly overlapped by the cervical vertebrae.

Figure 3. Skull of the holotype (MHGU-3000) of Musivavis amabilis n. gen. n. sp. in ventrolateral view. (1) photograph; (2) line drawing; (3) close-up of the rostral portion; (4) micro-CT scan of the rostral portion. Abbreviations: an, angular; dt, dentary teeth; fo, frontal fossa; fp, frontal process; fr, frontal; hy, hyoid; ju, jugal; la, lacrimal; ld, left dentary; mt, maxillary teeth; n, nasal; pmt, premaxillary teeth; pr, premaxilla; qu, quadrate; rd, right dentary; rm, right maxilla; sr, scleral ring; su, surangular; te, teeth.
The two dentaries are not fused at the symphysial area. The rostral end of the dentary tapers rostrodorsally. In its rostral two-thirds, the ventral margin of the dentary is straight, while the caudal one-third is slightly curved and tapered caudoventrally, as in other enantiornithines (Chiappe et al., Reference Chiappe, Meng, Serrano, Sigurdsen, Wang, Bell and Liu2019). A series of nutrient foramina is visible on the rostral part of the lateral surface of the right dentary. The right angular is robust, with tapered rostral end and expanded caudal end, measuring ~75% the length of the right dentary. The right surangular is preserved in lateral view and is shorter than the right angular, with extremely tapered rostral and caudal ends, and dorsally expanded middle part. The left surangular is articulated with the left dentary. The hyoids are straight and slender.
Teeth are present in the premaxilla, maxilla, and dentary (Fig. 3). As in most enantiornithines, the premaxillae possess four teeth, which are small (< 1 mm) and subconical in shape. The tooth root is rectangular-like in lateral view and nearly the same height as the crown. There is a slight constriction between the root and crown. The crown slightly expands basally and becomes sharp apically with the caudally recurved labial side and the relatively straight lingual side. The premaxillary teeth are most similar in size and morphology to the teeth of Eoenantiornis (Zhou et al., Reference Zhou, Chiappe and Zhang2005). They strongly differ from the large robust teeth in bohaiornithids, the large recurved and laterally compressed teeth of Longipteryx, and the numerous small and blunt teeth of Pengornis (O'Connor et al., Reference O'Connor, Zhang, Chiappe, Meng, Li and Liu2013). The interalveolar space is nearly equal to the crown width. Six teeth are visible in the right maxilla. The completely exposed maxillary teeth are much larger than the premaxillary teeth. The dentary teeth are smaller than the premaxillary and maxillary teeth but similar in morphology.
The cervical vertebrae are partially disarticulated (Fig. 4). The anterior four cervical vertebrae are preserved between the skull and the right coracoid. Three cervical vertebrae are preserved above the skull, and two additional cervical vertebrae are preserved near the furcula and the left coracoid. The cervical vertebrae are short and robust, with prominent ventral keels and zygapophyses. Three thoracic vertebrae are visible. These exhibit rectangular centra and distinctly concave posterior intercentral facets, as in most enantiornithines (Chiappe and Walker, Reference Chiappe, Walker, Chiappe and Witmer2002). The synsacrum is completely fused and includes at least seven vertebrae, with no more-detailed features that can be observed due to poor preservation (Fig. 5). The free caudal vertebrae are poorly preserved. The pygostyle is preserved in latero-ventral view and abruptly tapers distally (Fig. 5), similar to the shape of the distal end in Gretcheniao (Chiappe et al., Reference Chiappe, Meng, Serrano, Sigurdsen, Wang, Bell and Liu2019), and in contrast to the gently tapered distal end in other bohaiornithids (M. Wang et al., Reference Wang, O'Connor, Zheng, Wang, Hu and Zhou2014), and different from the more conspicuous constrictions in most enantiornithine pygostyles (e.g., Boluochia, Shanweiniao, and Rapaxavis; Zhou, Reference Zhou1995; O'Connor et al., Reference O'Connor, Wang, Chiappe, Gao, Meng, Cheng and Liu2009, Reference O'Connor, Chiappe, Gao and Zhao2011). The exact length of the pygostyle cannot be determined due to the broken proximal end. Two thoracic ribs are preserved next to the left humerus, which extend dorsally, defining an obtuse angle with the corresponding straight shaft (Fig. 6), differing from the acute angle in Parabohaiornis (M. Wang et al., Reference Wang, O'Connor, Zheng, Wang, Hu and Zhou2014). Six sternal ribs are preserved adjacent to the right side of the sternum. The middle three sternal ribs are longer and more recurved than the cranial and caudal sternal ribs. Six sternal ribs are also preserved close to the left side of the sternum, but are partially overlapped by the left scapula or are broken.

Figure 4. Cervical vertebrae of the holotype (MHGU-3000) of Musivavis amabilis n. gen. n. sp.

Figure 5. Synsacrum, pygostyle, and pubis of the holotype (MHGU-3000) of Musivavis amabilis n. gen. n. sp. (1) photograph of synsacrum; (2) photograph of pygostyle; (3) CT scan.

Figure 6. Pectoral girdle and sternum of the holotype of Musivavis amabilis n. gen. n. sp. (MHGU-3000). (1) photograph; (2) close-up of the furcula and coracoid; (3) close-up of the cranial margin of the sternum; (4) close-up of the xiphoid process of the sternum. Abbreviations: ac, acromial process of the scapula; ap, acrocoracoid process of the coracoid; clp, craniolateral process of the sternum; dr, dorsal ribs; fu, furcula; gf, glenoid facet of the scapula; imt, intermediate trabecula; lc, left coracoid; ls, left scapula; lt, lateral trabecula of the sternum; no, notch of the sternum; rc, right coracoid; rs, right scapula; sf, scapular facet; sp, supracoracoidal nerve foramen; st, sternum; str, sternal ribs; su, sulci of the furcular rami; thr, thoracic ribs; xp, xiphoid process of the sternum.
Both coracoids are preserved in dorsomedial view and are strut-like (Figs. 6, 7). The supracoracoid nerve foramen is visible in the right coracoid. The foramen is placed inside a distinct fossa excavating the medial margin of the coracoid. The acrocoracoid process is well developed and rectangular, with the long axis aligned with the long axis of the coracoid shaft. The coracoid bears a prominent tubercle forming the convex scapular facet, a feature present in the majority of enantiornithines (Chiappe and Walker, Reference Chiappe, Walker, Chiappe and Witmer2002; M. Wang et al., Reference Wang, O'Connor, Zheng, Wang, Hu and Zhou2014). The scapular facet prominently projects medially. CT scans reveal the sternal margin of the coracoid, which is relatively concave and slightly expanded mediolaterally (Fig. 7; Supplementary data 3, 4): the ratio of sternal margin width to coracoid shaft length is 44%. This condition is similar to most bohaiornithids but different from that of Gretcheniao, in which the sternal margin is less expanded mediolaterally (Fig. 8) (M. Wang et al., Reference Wang, O'Connor, Zheng, Wang, Hu and Zhou2014; Chiappe et al., Reference Chiappe, Meng, Serrano, Sigurdsen, Wang, Bell and Liu2019; Hu et al., Reference Hu, O'Connor, Wang, Wroe and McDonald2020). The scapula is straight and long, nearly 87% the length of the humerus. The acromial process is well developed and aligned to the long axis of the scapular shaft. The glenoid fossa is prominently concave and oriented laterally. The scapular morphology is similar to those of most bohaiornithids, Eoenantiornis, and Pterygornis (M. Wang et al., Reference Wang, Hu and Li2016), but different from the sagittally curved scapula of Zhouornis, Fortunguavis, and Dunhuangia (M. Wang et al., Reference Wang, Liu, O'Connor, Zhou and You2015), and from the laterally projected acromial process of Gretcheniao (Chiappe et al., Reference Chiappe, Meng, Serrano, Sigurdsen, Wang, Bell and Liu2019). A shallow elliptical fossa excavates longitudinally the distal half of the lateral surface of the scapula. CT scan reveals that the furcula is Y-shaped with a prominent hypocleideum, nearly 70% the length of the furcular ramus (Fig. 7; Supplementary data 5, 6). The hypocleideum is comparable in extent to other enantiornithines, being shorter than the furcular ramus, and gradually tapered distally. The epicleideal rami are relatively straight, with expanded and robust omal ends, forming an angle of ~45° at the junction, a value which is the same as that of Gretcheniao and Zhouornis, and smaller than that of Shenqiornis (50°), Sulcavis (60°), Bohaiornis (60°), and Parabohaiornis (60°). The expanded omal ends of the furcular rami are also consistent with those in Bohaiornithidae, but different from the tapered omal ends of Gretcheniao and most enantiornithines (Chiappe et al., Reference Chiappe, Meng, Serrano, Sigurdsen, Wang, Bell and Liu2019). The right furcular ramus, which is exposed in visceral view, shows a deep fusiform sulcus running along the whole ramus and extending onto the proximal third of the hypocleideum (Fig. 7; Supplementary data 5, 6). In other enantiornithines, the sulcus is either shallower or less expanded distally, not reaching the hypocleidum (e.g., Parapengornis; Hu et al., Reference Hu, O'Connor and Zhou2015). The sulci on both furcular rami are clearly visible in the CT scans (Fig. 7; Supplementary data 5, 6). A sharp keel runs along the ventral surface of the furcula, from the junction of the epicleideal rami to the distal tip of the hypocleideum (Supplementary data 5, 6).

Figure 7. CT renderings of the coracoid and furcula of the holotype (MHGU-3000) of Musivavis amabilis n. gen. n. sp. (1) Left coracoid in dorsomedial view; (2) left coracoid in ventromedial view; (3) right coracoid in lateromedial view; (4) right coracoid in ventromedial view; (5) furcula in dorsal view; (6) furcula in ventral view.

Figure 8. Comparative line drawings of coracoid of the holotype of Musivavis amabilis n. gen. n. sp. (MHGU-3000) and selected Mesozoic birds. (1) Musivavis amabilis n. gen. n. sp.; (2) Protopteryx fengningensis Zhang and Zhou, Reference Zhang and Zhou2000; (3) Kompsornis longicaudus X.-R. Wang et al., Reference Wang, Huang, Kundrát, Cau, Liu, Wang and Ju2020; (4) Abitusavis lii M. Wang et al., Reference Wang, Li, Liu and Zhou2020; (5) Bohaiornis guoi Hu et al., Reference Hu, Li, Hou and Xu2011; (6) Parabohaiornis martini M. Wang et al., Reference Wang, O'Connor, Zheng, Wang, Hu and Zhou2014; (7) Longusunguis kurochkini M. Wang et al., Reference Wang, O'Connor, Zheng, Wang, Hu and Zhou2014; (8) Gretcheniao sinensis Chiappe et al., Reference Chiappe, Meng, Serrano, Sigurdsen, Wang, Bell and Liu2019; (9) Sulcavis geeorum O'Connor et al., Reference O'Connor, Zhang, Chiappe, Meng, Li and Liu2013; (10) Shenqiornis mengi X.-R. Wang et al., Reference Wang, O'Connor, Zhao, Chiappe, Gao and Cheng2010; (11) Eoenantiornis buhleri Hou et al., Reference Hou, Martin, Zhou and Feduccia1999; (12) Eocathayornis walkeri Zhou, Reference Zhou2002.
The sternal plate, exposed in dorsal (visceral) view, is short and broad, with the width and length subequal (Fig. 6), as in other members of Enantiornithes (Chiappe and Walker, Reference Chiappe, Walker, Chiappe and Witmer2002). The cranial margin is flat at midline and curved near the craniolateral process. In other bohaiornithid-like taxa, craniolateral processes are present in Zhouornis (Zhang et al., Reference Zhang, Chiappe, Han and Chinsamy2013), but absent in Bohaiornis, Parabohaiornis, and Longusunguis (M. Wang et al., Reference Wang, O'Connor, Zheng, Wang, Hu and Zhou2014). Furthermore, the process extends craniolaterally and describes a nearly right angle, different from the laterally projected craniolateral process of Zhouornis, but similar to that of Pterygornis and Shangyang (M. Wang et al., Reference Wang, Hu and Li2016; Wang and Zhou, Reference Wang and Zhou2019). Craniolateral processes are hypothesized to ossify from separate centers and develop late in certain taxa (e.g., Rapaxavis and Concornis; M. Wang et al., Reference Wang, Liu, O'Connor, Zhou and You2015), which suggests that Musivavis n. gen. was not juvenile at the time of death. There is a very small notch on the lateral margin, similar to the condition in Longusunguis and Zhouornis, but different from the smooth lateral margin in Bohaiornis and Parabohaiornis (Hu et al., Reference Hu, O'Connor, Wang, Wroe and McDonald2020). The lateral trabecula is oriented caudolaterally and expands into a triangular process distally that expands more medially (resulting in a more acuminate medial corner) than laterally, a morphology consistent with most bohaiornithids (except for Longusunguis) and several Cathayornithidae (e.g., Cathayornis, Eocathayornis, and Houornis) (M. Wang et al., Reference Wang, Liu, O'Connor, Zhou and You2015). As in Bohaiornis, Eocathayornis, Shanweiniao, and Longirostravis, the xiphoid process extends nearly to the same level as the distal end of lateral trabecula and slightly expands transversely to be a blunt distal end (Zhang et al., Reference Zhang, Chiappe, Han and Chinsamy2013). The xiphoid process projects farther caudally than the lateral trabecula and flares slightly mediolaterally at the distal end in Parabohaiornis (M. Wang et al., Reference Wang, O'Connor, Zheng, Wang, Hu and Zhou2014). In other enantiornithines, the xiphoid process is tapered distally (e.g., Cathayornis, Houornis, Eoenantiornis, Longipteryx, and Rapaxavis; Zhang et al., Reference Zhang, Chiappe, Han and Chinsamy2013; M. Wang et al., Reference Wang, Liu, O'Connor, Zhou and You2015). As in Bohaiornis, Pterygornis, Cathayornis, and Eoenantiornis, the intermediate trabecula is short and oriented mediocaudally, contrary to the laterocaudally oriented intermediate trabecula in Parabohaiornis (M. Wang et al., Reference Wang, O'Connor, Zheng, Wang, Hu and Zhou2014) (Fig. 9).

Figure 9. Comparative line drawings of sternum of the holotype (MHGU-3000) of Musivavis amabilis n. gen. n. sp. and selected enantiornithines. (1) Musivavis amabilis n. gen. n. sp.; (2) Bohaiornis guoi; (3) Parabohaiornis martini; (4) Longusunguis kurochkini; (5) Zhouornis hani Zhang et al., Reference Zhang, Chiappe, Han and Chinsamy2013; (6) Cathayornis yandica Zhou et al., Reference Zhou, Jin and Zhang1992; (7) Eocathayornis walkeri; (8) Houornis caudatus Wang and Liu, Reference Wang and Liu2016.
The left forelimb is nearly complete and articulated (Fig. 10). It is nearly the same length as the hindlimb ([humerus + ulna + carpometacarpus]/[femur + tibiotarsus + metatarsal III] = 1.0). The intermembral index is similar to those of Gretcheniao (1.1) and Bohaiornithidae (0.9–1.1) (Chiappe et al., Reference Chiappe, Meng, Serrano, Sigurdsen, Wang, Bell and Liu2019; Hu et al., Reference Hu, O'Connor, Wang, Wroe and McDonald2020), a smaller value than that of other enantiornithine groups (e.g., >1.3 in Pengornithidae and ~1.2 in Longipterygidae) (X.-R. Wang et al., Reference Wang, Liu, O'Connor, Zhou and You2015; Hu et al., Reference Hu, O'Connor, Wang, Wroe and McDonald2020).

Figure 10. Left forelimb of the holotype (MHGU-3000) of Musivavis amabilis n. gen. n. sp. in ventral view. (1) Photograph; (2) close-up of the proximal ends of the left metacarpals; (3) close-up of the proximal ends of the right metacarpals. Abbreviations: al, alular metacarpal; ald-1–2, alular digit 1–2; bi, bicipital crest; hu, humerus; ma, major metacarpal; mad-1–3, major manual digit 1–3; mi, minor metacarpal; mid-1, minor manual digit 1; ra, radius; ul, ulna; se, semilunate carpal.
The humerus is slightly S-shaped and shorter than the ulna and radius. Proximally, the deltopectoral crest expands dorsally and decreases caudally at the first one-third of the humeral shaft. A prominent excavation runs along the dorsal margin of the deltopectoral crest. The bicipital crest is robust and projects anteroventrally, forming a deep notch with the humeral shaft (Fig. 10). The bicipital crest is not excavated by any fossa or foramen; in contrast, a large and deep fossa excavates the bicipital crest in Gretcheniao and Linyiornis (Chiappe et al., Reference Chiappe, Meng, Serrano, Sigurdsen, Wang, Bell and Liu2019; Y. Wang et al., Reference Wang, Wang, O'Connor, Wang, Zheng and Zhang2016). Distally, the dorsal condyle is broad and projects almost perpendicular to the long axis of the humeral shaft. The ventral condyle is not as developed as the dorsal condyle, only slightly extended beyond the distal end of the humerus. A deep and wide notch separates the dorsal and ventral condyles. The ulna is almost the same length as the radius, but considerably more robust and wider than the latter. The proximal part of the ulna is slightly curved, while the distal part is straight. The radius is relatively gracile and straight. The distal end of the radius seems slightly expanded. The carpals are not fused with the metacarpals. The semilunate carpal is preserved between the ulna and the minor metacarpal. The ulnare overlaps the radiale, preventing a detailed description of size and morphology of the latter.
As in other bohaiornithid-like taxa, the alular metacarpal is sub-rectangular in shape and ~29% the length of the major metacarpal (M. Wang et al., Reference Wang, O'Connor, Zheng, Wang, Hu and Zhou2014; Chiappe et al., Reference Chiappe, Meng, Serrano, Sigurdsen, Wang, Bell and Liu2019). The proximal ends of the major and minor metacarpals are completely fused together (Fig. 10). The major metacarpal, the most robust element in the metacarpus, is straight and uniformly wide. The minor metacarpal is nearly straight and extends farther distally than the major metacarpal, as in all enantiornithines (Chiappe and Walker, Reference Chiappe, Walker, Chiappe and Witmer2002). The relatively straight minor metacarpal and the narrow intermetacarpal space are similar to the condition in Longipteryx, Gretcheniao, and Bohaiornithidae, but different from other enantiornithines, which bear a more curved minor metacarpal forming the lateral margin of a relatively expanded intermetacarpal space (e.g., Hebeiornis and Eoenantiornis; M. Wang et al., Reference Wang, O'Connor, Zheng, Wang, Hu and Zhou2014; Chiappe et al., Reference Chiappe, Meng, Serrano, Sigurdsen, Wang, Bell and Liu2019; Hu et al., Reference Hu, O'Connor, Wang, Wroe and McDonald2020).
The phalangeal formula of the manus is thus 2-3-1-X-X, consistent with that of Cathayornithidae, Eoenantiornis, Gretctheniao, and Bohaiornithidae (Zhou et al., Reference Zhou, Chiappe and Zhang2005; O'Connor and Dyke, Reference O'Connor, Dyke, Boles and Worthy2010; Chiappe et al., Reference Chiappe, Meng, Serrano, Sigurdsen, Wang, Bell and Liu2019; Hu et al., Reference Hu, O'Connor, Wang, Wroe and McDonald2020). As in most enantiornithines, but unlike the bohaiornithids, Protopteryx, Longipteryx, Eoenantiornis, and Monoenantiornis (Zhou et al., Reference Zhou, Chiappe and Zhang2005; Hu and O'Connor, Reference Hu and O'Connor2017; Hu et al., Reference Hu, O'Connor, Wang, Wroe and McDonald2020), the alular digit terminates at two-thirds of the major metacarpal shaft. The shaft of the first phalanx of the alular digit is straight and slender. The proximal end of the phalanx expands transversely, resulting in a prominent medial corner, which describes a gentle concavity with the medial margin of the phalanx shaft. The alular ungual phalanx is smaller than the major ungual phalanx and not as curved as the latter. In the bohaiornithids, the alular ungual phalanx is larger than or equal to the major ungual phalanx (M. Wang et al., Reference Wang, O'Connor, Zheng, Wang, Hu and Zhou2014; Hu et al., Reference Hu, O'Connor, Wang, Wroe and McDonald2020). The first phalanx of the major manual digit is straight and as wide as the major metacarpal, which is also the longest phalanx. The second phalanx of the major manual digit is almost the same length of the first phalanx of the alular manual digit, but much wider and more robust than the latter. Only the first phalanx of the minor manual digit is preserved.
The pelvic elements are not well preserved. Only the proximal end of the ilium and the distal end of the ischium are visible, but few anatomical characteristics can be determined. CT scan reveals that the pubis is long, curved at the middle portion, and bears a distal boot expanded caudodorsally (Fig. 5). The femur is straight and 82% the length of the tibiotarsus, which is similar to most enantiornithines, but less than the pengornithids (where it is >90% tibiotarsus length; Hu and O'Connor, Reference Hu and O'Connor2017). The femoral head is prominent and projects almost perpendicular to the long axis of the femoral shaft. Distally, the lateral and medial condyles are well developed and separated by a deep notch. The tibiotarsus is long and robust, nearly the same in width as the femur. Only the proximal ends of both fibulae are preserved, and it is difficult to determine the exact length and the extent of the distal end.
The distal tarsals are fused to the proximal ends of metatarsals II–IV (Fig. 11). Metatarsal Ⅰ is short (21% the length of metatarsal Ⅲ) and terminates as a rounded articular facet at the proximal facet of the trochlea of metatarsal Ⅱ. Metatarsals Ⅱ–Ⅳ are completely fused proximally. Metatarsal Ⅲ is the longest and metatarsal Ⅱ is slightly shorter than metatarsal Ⅲ and metatarsal Ⅳ. As in most enantiornithines, metatarsal Ⅳ is much thinner than metatarsals Ⅱ–Ⅲ. The pedal phalangeal formula is 2-3-4-5-X. The first pedal digit is reversed compared with other digits. The second pedal digit is the most robust toe, as in other bohaiornithid-grade taxa (M. Wang et al., Reference Wang, O'Connor, Zheng, Wang, Hu and Zhou2014), while the third is the longest. All phalanges have well-developed proximal flexor processes. All pedal digits possess sharp and moderately recurved ungual phalanges. Each pedal ungual phalanx bears prominent neurovascular sulci and shows a distinct horny claw. The fourth pedal ungual phalanx is smaller than the other three pedal digit ungual phalanges, a condition approaching that in bohaiornithids (M. Wang et al., Reference Wang, O'Connor, Zheng, Wang, Hu and Zhou2014). The first pedal ungual phalanx is the most robust ungual phalanx and nearly equal in length to the second and third ungual phalanges, a condition which is different from that in other bohaiornithids, where the third ungual phalanx is the longest, recalling the condition in pengornithids (M. Wang et al., Reference Wang, O'Connor, Zheng, Wang, Hu and Zhou2014; Hu et al., Reference Hu, O'Connor and Zhou2015, Reference Hu, O'Connor, Wang, Wroe and McDonald2020).

Figure 11. Feet of the holotype (MHGU-3000) of Musivavis amabilis n. gen. n. sp. (1) Photograph; (2) close-up of the proximal end of the left tarsometatarsus; (3) close-up of the proximal end of the right tarsometatarsus. Abbreviations: mt-I–IV, metatarsal I–IV; I-1–2, pedal digit I-1-2; II-1–2–3, pedal digit II-1–2–3; III-3–4, pedal digit III-3–4; 1–2–3–4–5, pedal digit IV-1–2–3–4–5.
Etymology
The specific name is derived from the Latin “amabilis” (lovely or beautiful), referring to the exquisite preservation of the holotype.
Remarks
Musivavis amabilis n. gen. n. sp. is much smaller in size than currently known bohaiornithids and pengornithids, and differs from the comparably sized cathayornithids (e.g., Cathayornis, Hebeiornis, Sinornis) in the following features: omal tips of furcula transversely expanded; sternum with craniolateral processes and expanded, triangular-shaped distal end of the lateral trabecula (both features absent in Hebeiornis); deltopectoral crest less prominent relative to humerus shaft (more developed in Cathayornis and Sinornis); minor metacarpal not strongly bowed posteriorly, resulting in a slit-like intermetacarpal space (distinctly bowed in Cathayornis and Sinornis, resulting in a larger intermetacarpal space); ungual phalanx of major digit more curved than the alular ungual phalanx (less curved in Hebeiornis); distal end of metatarsal II subequal in width to distal end of metatarsal III (wider in Hebeiornis and Sinornis); poorly developed flexor tubercles in the pedal ungual phalanges (more prominent in Hebeiornis and Sinornis).
Osteohistological assessment of the ontogenetic age
Bone tissue of the left tibiotarsus midshaft was sampled to determine the ontogenetic age of the holotype of Musivavis amabilis n. gen. n. sp. (MHGU-3000). A transverse section made through the sample exhibits two different kinds of the bone tissue: cortical bone and endosteal bone (Fig. 12).
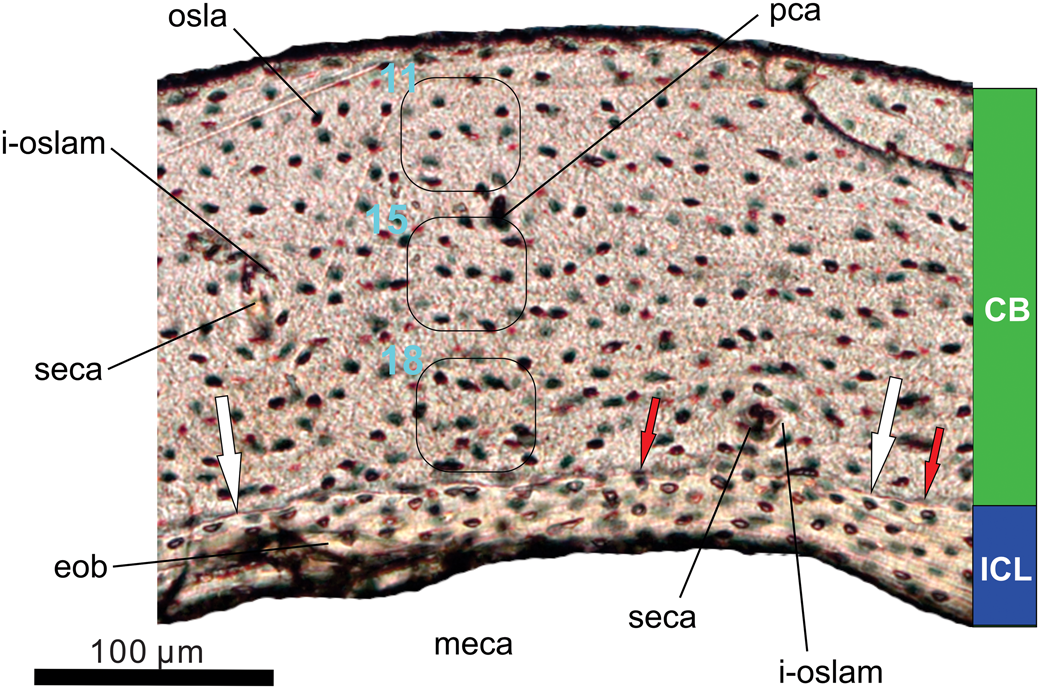
Figure 12. Histology of the left tibiotarsus of the holotype (MHGU-3000) of Musivavis amabilis n. gen. n. sp. Transverse section of the midshaft level is viewed in transmitted light. Note decreasing counts of osteocyte lacunae per area, for example from 18 in the innermost cortex to 11 in the outermost cortex (outlined areas in CB). The white arrows point an irregular interface between the cortical and endosteal bones, whereas the red arrows mark partially eroded osteocyte lacunae. Abbreviations: CB, cortical bone; eob, endosteal bone; ICL, inner circumferential layer; i-oslam, ill-developed osteonal lamellae; meca, medullary cavity; osla, osteocyte lacuna; pca, primary osteonal (neurovasular) canal; seca, secondary osteonal canal.
The cortical bone (CB) is composed of a thicker (172–186 μm) layer of poorly vascularized bone resembling tissue, which has been considered as parallel-fibered bone in previously described enantiornithines (Chinsamy et al., Reference Chinsamy, Chiappe and Dodson1995; M. Wang et al., Reference Wang, O'Connor, Pan and Zhou2017). A few secondary osteonal canals are present and may indicate an initial reconstruction of the primary bone. However, circumferential osteonal lamellae associated with the canals are rather poorly developed. No outer circumferential layer (OCL) has been recognized in the sample of Musivavis amabilis n. gen. n. sp., indicating that active bone deposition had not ceased at the time of death (de Ricqlès et al., Reference de Ricqlès, Meunier, Castanet, Francillon-Vieillot and Hall1991).
The endosteal bone (EB) forms the compact avascular inner circumferential layer (ICL), enclosing a large hollowed medullary cavity. The endosteal bone is variably thick (minimum: 30 μm; maximum: 47 μm) and accounts for at least a fifth (~21%) of the total cortex. The cortical and endosteal bones are separated by a scalloping line visible on the section (see white arrows in Fig. 12). This line represents the erosional front. Its irregular shape corresponds to variable rates at which the primary bone was resorbed, and provides evidence about the removal of earliest bone formed in the specimen.
No resting marks, either annuli or lines of arrested growth (LAG), are developed in the sample. This may imply continuous growth in Musivavis n. gen. between hatching and death. We cannot rule out, however, that features associated with an early growth interruption were already removed by the bone resorption in the specimen. On the other hand, we may certainly reject the interpretation that the resorption line is a LAG, mainly due to partially eroded osteocyte lacunae being distributed along the erosional front (see red arrows in Fig. 12). Moreover, microlaminar texture and organized osteocyte lacunae characterize the EB, and these characters markedly differ from conditions seen in the CB of Musivavis n. gen.
Up to now, a wide range of developmental stages of enantiornithine birds have been examined osteohistologically including: (1) an embryo (Enantiornithes indet.: MUCPv-352-353) from the Late Cretaceous of Argentina (Schweitzer et al., Reference Schweitzer, Jackson, Chiappe, Schmidt, Calvo and Rubilar2002) and another embryonic enantiornithine (Gobipteryx minuta, Elzanowski, Reference Elzanowski1974: ZPALMgR ǁ/90) from the Late Cretaceous of Mongolia (Chinsamy and Elzanowski, Reference Chinsamy and Elzanowski2001); (2) an early juvenile (Enantiornithes indet.: MPCM-LH-26189, no LAGs) from the Early Cretaceous of Spain (Knoll et al., Reference Knoll, Chiappe, Sanchez, Garwood, Nicholas, Wogelius, Sellers, Manning, Ortega, Serrano, Marugán-Lobón, Cuesta, Escaso and Sanz2018); (3) a juvenile (Pengornithidae indet.: IVPP V15576, no LAGs) from the Early Cretaceous of China (O'Connor et al., Reference O'Connor, Erickson, Norell, Bailleul, Hu and Zhou2018); (4) a subadult (Cruralispennia multidonta M. Wang et al., Reference Wang, O'Connor, Pan and Zhou2017: IVPP V21711, no LAGs) from the Early Cretaceous of China (M. Wang et al., Reference Wang, O'Connor, Pan and Zhou2017) and another subadult individual (Patagopteryx deferrariisi Alvarenga and Bonaparte, Reference Alvarenga, Bonaparte and Campbell1992: MACN-N-03, one LAG plus one annulus) from the Late Cretaceous of Argentina (Chinsamy et al., Reference Chinsamy, Chiappe and Dodson1995); and (5) some adults from the Early Cretaceous of Spain (cf. Concornis lacustris Sanz and Buscalioni, Reference Sanz and Buscalioni1992: LH 21006, LAGs = 2; Cambra-Moo et al., Reference Cambra-Moo, Buscalioni, Cubo, Castanet, Loth, de Margerie and de Ricqlès2006) and from the Late Cretaceous of Argentina (Enantiornithes indet.: PVL-4273, five LAGs; Chinsamy et al., Reference Chinsamy, Chiappe and Dodson1995).
The absence of annulus or LAG and OCL suggests that the holotype individual was likely an early juvenile stage, whereas the presence of the relatively thick ICL, abundance of non-flattened osteocyte lacunae in the outer cortex, and occurrence of the poorly developed secondary osteons suggest a slightly later developmental age. Compared to other enantiornithines, the advanced degree of co-ossification in the elements of the sternum, carpometacarpus, tibiotarsus, and tarsometatarsus is equivalent to developmental “stage 3” of Hu and O'Connor (Reference Hu and O'Connor2017). Therefore, we suggest that the holotype of Musivavis amabilis n. gen. n. sp. perished as a subadult near the ontogenetic stage characterized by the first growth deceleration.
Results of the phylogenetic analyses
The phylogenetic analysis setting all characters as equally weighted found 6400 shortest trees of 1920 steps each (Consistency Index excluding uniformative characters = 0.2765; Retention Index = 0.5131) and resolved MHGU-3000 as nested within a lineage that, in turn, is the sister taxon of the node including Bohaiornithidae and Pengornithidae (Fig. 13). The strict consensus of the shortest trees found shows that the relationships among Enantiornithes are well resolved but weakly supported—a result in agreement with previous studies based on independently developed data sets (e.g., M. Wang et al., Reference Wang, O'Connor, Zheng, Wang, Hu and Zhou2014). In particular, the analysis supports the monophyly of Longipterygidae, Pengornithidae, and Avisauridae. Musivavis n. gen. was found as more closely related to Dunhuangia (M. Wang et al., Reference Wang, Liu, O'Connor, Zhou and You2015) and Longusunguis (M. Wang et al., Reference Wang, O'Connor, Zheng, Wang, Hu and Zhou2014) than to other enantiornithines, in a lineage that also includes Houornis (Wang and Liu, Reference Wang and Liu2016) and, tentatively, Yungavolucris (the latter acts as a “wildcard” taxon and might instead be an avisaurid; Chiappe and Walker, Reference Chiappe, Walker, Chiappe and Witmer2002). Characters supporting this clade are the straight and elongated frontal process of premaxilla, the dentary with subparallel dorsal and ventral margins along its occlusal part, the expanded omal tips of the furcula, and the strongly curved second pedal ungual phalanx. This clade shares with the “Bohaiornithidae + Pengornithidae” clade a relatively stout first phalanx of the second pedal digit and the more proximal position of the trochlea of metatarsal II relative to those of metatarsal III and IV. These features are relatively homoplastic among Pygostylia (mean homoplasy index = 0.74) and did not support this clade when the dataset was re-analyzed using the “Extended Implied Weighting” function (Fig. 14; Supplementary data 7). When K is set as = 5 or = 10 (most aggressive downweighting settings of the homoplastic characters among those tested), the pengornithids are reconstructed among the earliest-diverging enantiornithine lineages, not closely related to the “bohaiornithid-grade” forms. Under the less aggressive K values used, (i.e., 15 and 20), pengornithids form a more derived enantiornithine lineage, relatively closer to the “bohaiornithid-grade” forms. All analyses using the “Extended Implied Weighting” function reconstructed Musivavis n. gen. as sister group of Dunhuangia, with the two taxa consistently recovered among the “bohaornithid-grade” group, but nested inside Bohaiornithidae (sensu stricto, M. Wang et al., Reference Wang, O'Connor, Zheng, Wang, Hu and Zhou2014) only when K = 15.
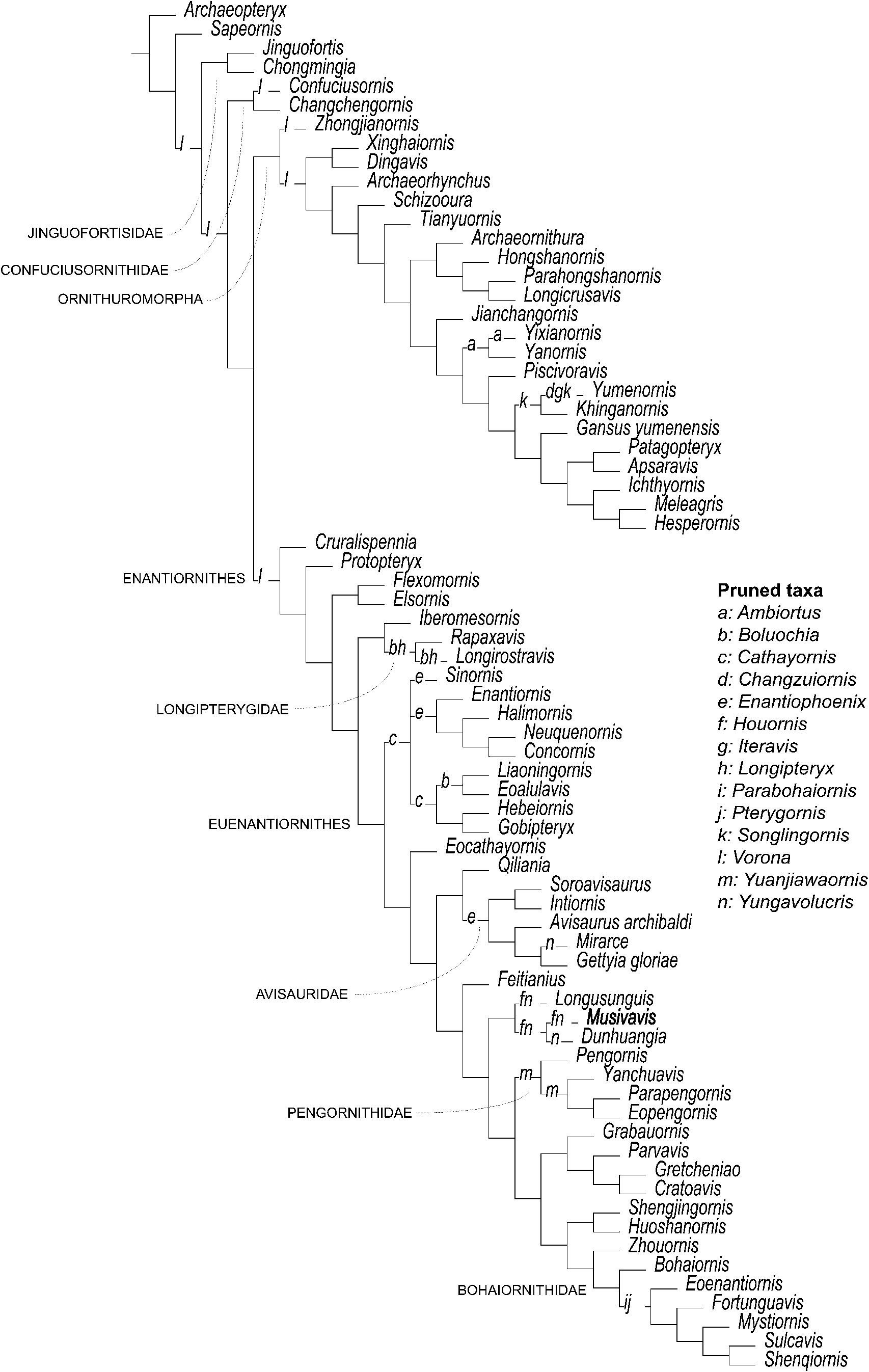
Figure 13. Reduced strict consensus of the shortest trees found by the unweighted analysis after pruning of the “wildcard” taxa. Letters at branches indicate the alternative placements of the “wildcard” taxa.

Figure 14. The effect of homoplastic characters in enantiornithine relationships. Two alternative topologies obtained setting an aggressive (left) or moderate (right) downweighting of the characters according to their homoplasy. Note, in particular, the radically different placements of the pengornithids (blue branches) under the two alternative weighting settings. On the contrary, Musivavis n. gen. (bold) is consistently found among the least inclusive clade containing the bohaiornithid-like taxa (red). Avisauridae, Longipterygidae, and Ornithuromorpha collapsed for brevity.
Discussion
The completely fused synsacrum, the extensive fusion among the elements in the sternum, carpometacarpus, tibiotarsus and tarsometatarsus, and the smooth external surface of the long bones (i.e, lacking a wrinkled texture) indicate that Musivavis n. gen. is more mature skeletally than other recently described juvenile enantiornithines (Wang et al., Reference Wang, Stidham, Li, Xu and Zhou2021). The osteohistological analyses also suggest that MHGU-3000 perished during subadult ontogeny. Musivavis n. gen. shows typical synapomorphies of Enantiornithes, such as the sternum with nearly equal length and width, the “Y”-shaped furcula with a long hypocleideum, the minor metacarpal extending farther distally than the major metacarpal, and the fourth metatarsal relatively thin (Chiappe and Walker, Reference Chiappe, Walker, Chiappe and Witmer2002).
The combination of features in Musivavis n. gen. is unique among presently known enantiornithines, supporting the erection of a new taxon. Musivavis n. gen. differs from the similar body-sized Cathayornis (Zhou et al., Reference Zhou, Jin and Zhang1992), Houornis (Hou, Reference Hou1997; O'Connor and Dyke, Reference O'Connor, Dyke, Boles and Worthy2010; Wang and Liu, Reference Wang and Liu2016), Hebeiornis (Xu et al., Reference Xu, Yang and Deng1999), Eoenantiornis (Zhou et al., Reference Zhou, Chiappe and Zhang2005), and Grabauornis (Dalsätt et al., Reference Dalsätt, Ericson and Zhou2014) in the morphology of the sternum. The deep notch on the lateral margin near the lateral trabecula of the sternum, which is shared by Cathayornis, Houornis, and Grabauornis, is different from the small notch near the craniolateral process in Musivavis n. gen. The xiphoid process extends farther distally than the lateral trabecula in Hebeiornis and Eoenantiornis, while they terminate almost at the same level in Musivavis n. gen. In particular, in most enantiornithines the sterna lack distinct craniolaterally projected craniolateral processes (only documented in Protopteryx, Pterygornis, and Shangyang; Wang and Zhou, Reference Wang and Zhou2019) and show a uniformly rounded/parabolic cranial margin (e.g., Protopteryx, Longipteryx, Cathayornis, Bohaiornis, and Pterygornis; M. Wang et al., Reference Wang, Liu, O'Connor, Zhou and You2015), differing from the relatively flat cranial margin in Musivavis n. gen. The distal expansion of the lateral trabecula and xiphoid process, combined with the size reduction of the alular digit and the more reduced phalangeal formula of the minor digit, further differentiate Musivavis n. gen. from Protopteryx (Zhang and Zhou, Reference Zhang and Zhou2000). The same features listed above, and the absence of the peculiar craniomandibular specializations of the longipterygids, preclude the referral of Musivavis n. gen. to the latter clade (Zhang et al., Reference Zhang, Zhou, Hou and Gu2001). Musivavis n. gen. lacks the brachyodont unrecurved teeth and the stouter metatarsus and hallux, which are diagnostic features for Pengornis and related taxa (Hu et al., Reference Hu, O'Connor and Zhou2015), and clearly differs from the latter in the morphology of the sternum.
Musivavis n. gen. resembles Bohaiornithidae in possessing several diagnostic characteristics of this family, such as the subconical teeth with tapered and slightly caudally recurved tips, the sternum with lateral trabecula strongly projected caudolaterally, and the omal end of the furcular ramus with blunt expansion (M. Wang et al., Reference Wang, O'Connor, Zheng, Wang, Hu and Zhou2014). Our phylogenetic analyses resolved Musivavis n. gen. as consistently nested within the group including the “bohaiornithid-like” taxa (Figs. 13, 14). Furthermore, the “bohaiornithid-like” features of Gretcheniao, discussed by Chiappe et al. (Reference Chiappe, Meng, Serrano, Sigurdsen, Wang, Bell and Liu2019), result as genuine synapomorphies shared by Gretcheniao and the “core bohaiornithids.” Musivavis n. gen. is much smaller than both Gretcheniao (ratio of humerus length = 0.6) and the known bohaiornithids (ratio of humerus length ranges from 0.58–0.8) (Table 2). Bohaiornithidae is diagnosed by, among others, the presence of large subconical teeth (width of the basal part of tooth crown varies from 0.72–0.98 mm), and strongly curved and elongated pedal ungual phalanges (i.e., the third pedal ungual phalanx is at least half the length of the tarsometatarsus) (M. Wang et al., Reference Wang, O'Connor, Zheng, Wang, Hu and Zhou2014; Hu et al., Reference Hu, O'Connor, Wang, Wroe and McDonald2020). The teeth of Musivavis n. gen. are small (widest basal part of crown = 0.3 mm), but not as curved as those of other bohaiornithids. The third pedal ungual phalanx of Musivavis n. gen. is not significantly elongate, and likely retains the plesiomorphic condition of most enantiornithines. Both the smaller body size and the different tooth and pedal morphologies suggest that Musivavis n. gen. was ecologically distinct from the bohaiornithids.
Table 2. Measurements (in mm) of selected skeletal elements of bohaiornithids. Musivavis n. gen. was measured directly and the others were cited from M. Wang et al., Reference Wang, O'Connor, Zheng, Wang, Hu and Zhou2014, and Chiappe et al., Reference Chiappe, Meng, Serrano, Sigurdsen, Wang, Bell and Liu2019.

The phylogenetic relationships among the enantiornithines are particularly controversial (e.g., Chiappe and Walker, Reference Chiappe, Walker, Chiappe and Witmer2002; O'Connor et al., Reference O'Connor, Wang, Chiappe, Gao, Meng, Cheng and Liu2009; O'Connor and Chiappe, Reference O'Connor and Chiappe2011; Hu et al., Reference Hu, O'Connor and Zhou2015; Chiappe et al., Reference Chiappe, Meng, Serrano, Sigurdsen, Wang, Bell and Liu2019). Our analyses show that homoplasy has a significant effect on the relationships among the main lineages, in particular on the position of Pengornithidae (Hu et al., Reference Hu, O'Connor and Zhou2015). The latter group has been reconstructed alternatively as very basal enantiornithines (e.g., X.-L Wang et al., Reference Wang, O'Connor, Zheng, Wang, Hu and Zhou2014; Chiappe et al., Reference Chiappe, Meng, Serrano, Sigurdsen, Wang, Bell and Liu2019) or as relatively more-derived taxa (O'Connor et al., Reference O'Connor, Zhang, Chiappe, Meng, Li and Liu2013; M. Wang et al., Reference Wang, O'Connor, Zheng, Wang, Hu and Zhou2014; Hu et al., Reference Hu, O'Connor and Zhou2015). The alternative placements of the pengornithids among Enantiornithes tested in our analyses suggest that the scenarios reconstructing Pengornithidae among late-diverging groups (e.g., relatively close to Bohaiornithidae) are supported by the most-homoplastic characters, in particular those related to scansorial adaptations (i.e., size and proportions of the pedal phalanges). When the phylogenetic weight of those homoplastic characters is mitigated, pengornithids are instead reconstructed as early-diverging enantiornithines—a position that is more in agreement with the retention in these birds of several pygostylian plesiomorphies (e.g., presence of numerous teeth, a high brachial index, the relative elongation of the fibula, the presence of the fifth metatarsal, see X.-L Wang et al., Reference Wang, O'Connor, Zheng, Wang, Hu and Zhou2014; Hu et al., Reference Hu, O'Connor and Zhou2015). In spite of its “mosaic” morphology, the phylogenetic position of Musivasis was less affected by alternative assumptions about the weighting of homoplastic characters in the analysis. Musivavis n. gen. is consistently reconstructed among the “bohaiornithid-grade” group, although its peculiar morphology challenges it being nested among the “core” Bohaiornithidae (the least inclusive clade containing Bohaiornis and Shenqiornis; M. Wang et al., Reference Wang, O'Connor, Zheng, Wang, Hu and Zhou2014). This result is in agreement with the recent discovery of other “bohaiornithid-like” taxa which shows only a subset of the features diagnostic of Bohaiornithidae (e.g., Chiappe et al., Reference Chiappe, Meng, Serrano, Sigurdsen, Wang, Bell and Liu2019): these results suggest that the “core” bohaiornithids are part of a more speciose “bohaiornithoid” radiation, including both small-bodied taxa (e.g., Musivavis n. gen.) and large-bodied taxa (e.g., Gretcheniao).
Conclusions
The unique combination of features of MHGU-3000 supports erection of a new genus and new species of Enantiornithes, Musivavis amabilis n. gen. n. sp., which enriches the diversity of the Jehol Biota birds. The mosaic of anatomical characters in M. amabilis n. gen. n. sp. and its smaller body size (presuming that the immature specimen would have reached only a marginally larger adult body size) differentiate it from the large-sized bohaiornithid enantiornithines, suggesting that this taxon occupied an ecological niche distinct from those of its closest relatives. Under all alternative scenarios tested, Musivavis n. gen. was part of a relatively rich clade with a wide range of adult body sizes and trophic adaptations. The reconstruction of enantiornithine relationships is affected by homoplasy and skeletal modularity. The homoplasy in the appendicular modules (in particular, the recurrent evolution of scansorial adaptations in the foot) might have a significant effect on the relative placements of Bohaiornithidae and Pengornithidae. The consistent reconstruction of the “bohaiornithid-grade,” including Musivavis n. gen., under different downweighting settings supports the monophyly of this successful radiation of ecologically disparate enantiornithines.
Acknowledgments
We would like to thank M. Wang for preparing the specimen, K. Fang for preparing the histological sample, and S. Chen and P. Yin for conducting the CT scan and reconstructions. We also appreciate M. Pittman and three anonymous reviewers for their constructive comments on the early version of the manuscript. Many thanks go to D. Ksepka and J. Kastigar for their kind suggestions and attention. This work was supported by projects from the National Natural Science Foundation of China (41872018, 41672019), the China Geological Survey (DD20190602), the Scientific Grant Agency VEGA of the Ministry of the Education, Science, Research and Sport of the Slovak Republic (1/0075/22) and the Slovak Research and Development Agency (APVV-18-0251).
Data availability statement
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.5tb2rbp5k.



















