Introduction
The Ediacara biota (575–541 Myr) represent the first appearance of complex, macroscopic life on Earth, and body plan diversity spans a broad array from discs and tubes to quilted and fractal forms. Taxonomic affinities and life histories of many of these organisms remain either controversial or poorly understood. The genus Dickinsonia has been the subject of near-continual debate since it was first described 70 years ago (Sprigg, Reference Sprigg1947). Descriptive and molecular-informed studies have sought to place Dickinsonia into various metazoan groups, as well as other kingdoms. Interpretations range from a pelagic cnidarian (Sprigg, Reference Sprigg1947; Valentine, Reference Valentine1992) and polychaete worm (Glaessner and Wade, Reference Glaessner and Wade1966; Wade, Reference Wade1972) to benthic placozoan (Sperling and Vinther, Reference Sperling and Vinther2010) and ctenophore (Zhang and Reitner, Reference Zhang and Reitner2006). Taphonomic and morphological arguments have been used to place Dickinsonia in the proposed kingdom Vendobionta as a serially quilted unicellular organism (Seilacher et al., Reference Seilacher, Grazhdankin and Legouta2003), or as a ground-dwelling lichen (Retallack, Reference Retallack2007). Other studies have focused on Dickinsonia’s unique, serially repeating body plan to estimate oxygen requirements, most recently demonstrating that adequate oxygenation may have been achieved via surficial diffusion (see Runnegar, Reference Runnegar1982; Gooden, Reference Gooden2014). This body plan has also been utilized to suggest Dickinsonia was a bilaterian-grade animal, due to interpreted growth via the terminal addition of units from a posterior growth region (Runnegar, Reference Runnegar1982; Gold et al., Reference Gold, Runnegar, Gehling and Jacobs2015). Most recently, growth models have been employed to infer a eumetazoan affinity for Dickinsonia via studies suggesting both terminal (Evans et al., Reference Evans, Droser and Gehling2017) and pre-terminal (Hoekzema et al., Reference Hoekzema, Brasier, Dunn and Liu2017) addition of body units. The identification of resting traces associated with several individual specimens indicates that it may have fed on the underlying microbial mat substrate via basal surface absorption (Gehling et al., Reference Gehling, Droser, Jensen and Runnegar2005; Sperling and Vinther, Reference Sperling and Vinther2010; Ivantsov, Reference Ivantsov2011).
This study focuses on a population of Dickinsonia costata Sprigg, Reference Sprigg1947 from Crisp Gorge, South Australia (Fig. 1). The Crisp Gorge population comprises 53.5% of the total abundance on the semi-contiguous surface (Reid et al., Reference Reid, García-Bellido, Payne, Runnegar and Gehling2017), and provides an excellent opportunity to study this taxon in its juvenile form. The remainder of the community comprises seven taxa and the biogenic sedimentary structure ‘Mop’ (Tarhan et al., Reference Tarhan, Droser and Gehling2010). Three additional taxa on the surface are also interpreted as juvenile: Parvancorina minchami Glaessner, Reference Glaessner1958, Tribrachidium heraldicum Glaessner, Reference Glaessner1959, and a single specimen of Rugoconites enigmaticus Glaessner and Wade, Reference Glaessner and Wade1966. The Textured Organic Surface (TOS) components of the microbial mat substrate include a range of structures, including round, deep relief bosses and regions of TOS components including ‘groove’ and ‘weave’ (Gehling and Droser, Reference Gehling and Droser2009). The overall fossil surface, however, is relatively smooth, and is interpreted as immature (Reid et al., Reference Reid, García-Bellido, Payne, Runnegar and Gehling2017).
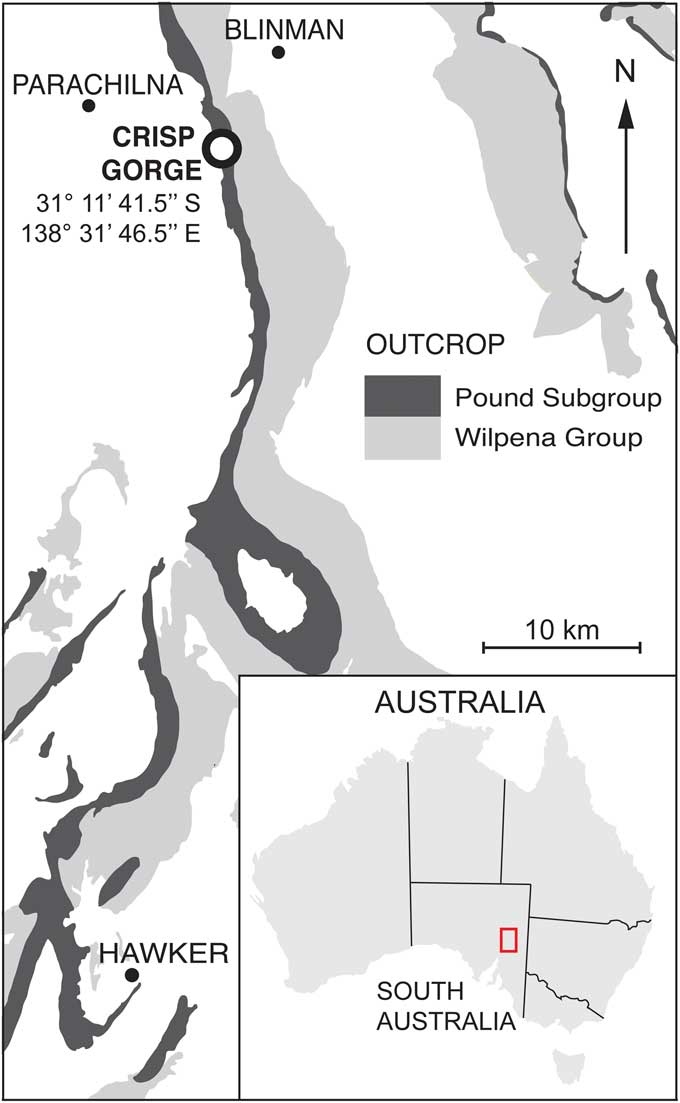
Figure 1 Locality map of the Flinders Ranges and Crisp Gorge fossil site. The Crisp Gorge fossil site is located within the Ediacara Member of the Pound Subgroup.
Geologic setting
In South Australia, the Ediacara Member marks the fossiliferous shallow marine, deltaic succession of the Rawnsley Quartzite. The Rawnsley Quartzite is the terminal package of Ediacaran-aged sediments within the Pound Subgroup of the Adelaide Rift Complex (Gehling and Droser, Reference Gehling and Droser2012). The Ediacara Member incorporates a number of fossiliferous facies, ranging from shore-face settings, to deeper water delta-front or pro-delta environments (Gehling and Droser, Reference Gehling and Droser2013). By far the richest, in situ, fossil assemblages are found within the Oscillation Rippled Sandstone facies (Tarhan et al., Reference Tarhan, Droser, Gehling and Dzaugis2017). These comprise medium- to coarse-grained feldspathic sands with distinctive rippled bed-tops, representative of a depositional environment between fair-weather and storm wave bases.
Locality information
The Crisp Gorge fossil locality (31°11'41.5"S, 138°31'46.5"E, Fig. 1) is primarily composed of repeating units of the Oscillation Rippled Sandstone facies (ORSF) and the deeper water Flat Laminated to Linguoid Rippled Sandstone facies, the latter indicative of a delta-front to pro-delta setting (Tarhan et al., Reference Tarhan, Droser, Gehling and Dzaugis2017). Both facies are present at this locality as interbedded dark red to maroon sands and silts, with the ORSF exhibiting characteristic bed-top combined-flow ripples and several bed partings revealing a silt layer up to 3 mm thick (Reid et al., Reference Reid, García-Bellido, Payne, Runnegar and Gehling2017). The majority of the sample material examined for this study was removed from the Crisp Gorge fossil locality and now forms a framed rock wall in the ‘First Life: Ediacara Biota Gallery’ of the South Australian Museum. This material is referred to as the ‘Crisp Wall’ throughout this study. The Crisp Wall surface is comprised of 16 semi-contiguous slabs of medium- to coarse-grained feldspathic sandstone from the ORSF and gives a surface area of ~6.5 m2. A further seven fossiliferous slabs determined to be semi-contiguous with the bedding surface of the existing Crisp Wall slabs have been identified at the Crisp Gorge fossil site and are incorporated into this study.
Materials and methods
The Dickinsonia costata specimens identified on the Crisp Wall, combined with the newly identified Crisp Gorge slabs, yielded a total of 150 specimens suitable for analysis. All measurements and observations were made from positive-relief, latex casts taken from the Crisp Wall specimens to aid identification of otherwise negative-relief features and allow for the use of a dissecting microscope. For the purpose of this study, phylogenetically neutral labels are used to describe a number of morphological features of the studied D. costata. This is to aid in the identification of features, while avoiding ambiguity or incidental taxonomic affinities that may be inferred from using body terms usually associated with animals. The terms ‘A-end’ and ‘B-end’ are used to refer to either end of the specimens as defined by the placement of a midline, with the A-end characterized by the terminal A-end unit (Fig. 2.1). The B-end is located at the opposite end of the body, and is defined by decreasing unit size (Fig. 2.1).
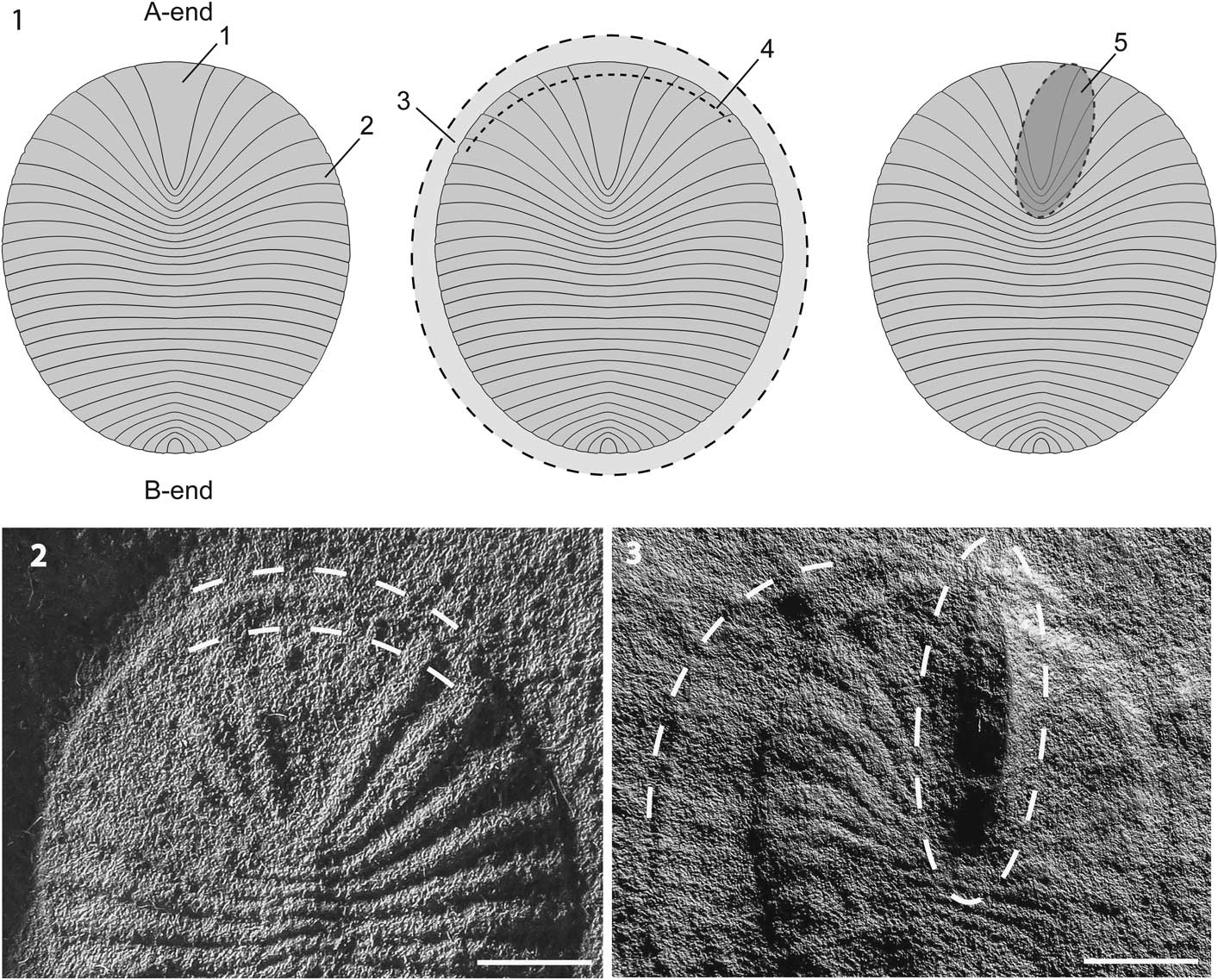
Figure 2 (1) Descriptive labels for Dickinsonia costata features: 1=terminal A-end unit, 2=body unit, 3=position of shrinkage rim, 4=position of A-end lip, 5=generalized position of A-end protuberance. (2) Detail of A-end lip feature, visible as a narrow, raised rim on the peripheral margin of the terminal A-end unit, SAMP34228. (3) Detail of shrinkage rim feature as an uneven margin exterior to the body fossil, and the A-end protuberance as a high relief, elongate structure involving the terminal A-end unit and several A-end units, SAMP34232. The surface visible is assumed to be the upper surface. Scale bars=3mm.
Measurements for each specimen included the maximum length, as the longest distance between A- and B-ends of the specimen, maximum width, taken as the widest measure perpendicular to the midline, and the number of units. Unit counts were not taken for individuals with damaged or indistinct units, defined here as those individuals with any form of mechanical damage to the rock or sand grains that mold them, and/or those that are indistinct from neighboring units. Measurements and unit counts were made with the aid of calipers and a dissecting microscope. Observations also were made of three body features, which have been termed the A-end lip, A-end protuberance, and shrinkage rim (see Results for details).
Statistical analysis of the D. costata population was performed using the open access statistical software program PAST (Hammer et al., Reference Hammer, Harper and Ryan2001). A Model II (major axis) regression was used in the analysis of body size (Legendre and Legendre, Reference Legendre and Legendre1998). A rose diagram of axial orientation was produced using the open access statistical software programs R (RCoreTeam, 2015) and RStudio (RStudioTeam, 2015), and the package ‘circular’ (Lund and Agostinelli, Reference Lund and Agostinelli2015). Pairwise correlations among three observed features (A-end lip, shrinkage rim, and A-end protuberance) were calculated using the Pearson method for the presence-absence data collected. Significance of relationships was calculated using Chi-squared tests with Yates’ continuity correction. To test if the D. costata size distribution was best described by a single or multiple normal distributions, we fitted univariate Gaussian finite mixture models to specimen length using the function ‘Mclust’ from the R package ‘mclust’ (Fraley et al., Reference Fraley, Raftery, Murphy and Scrucca2012), and compared these by optimization (minimization) of the Bayesian Information Criterion (BIC).
Repository and institutional abbreviation
The majority of specimens examined in this study are housed in the paleontology collections of the South Australia Museum, Adelaide, Australia (Prefix SAMP), and are currently on display in the “First Life: Ediacara Biota Gallery”. The remainder of specimens are located at the Crisp Gorge fossil locality. Specimens figured (Figs. 2, 4) are SAMP32351, SAMP34219, SAMP34224, SAMP34228, SAMP34232, SAMP34235, SAMP34243, SAMP34254 and SAMP34326. Please see Appendix A for the complete list of examined specimens and data set.
Results
Population size and shape
Dickinsonia costata is constructed of repeated, parabolic units intersecting a narrow medial axis. The terminal A-end unit forms a deep V-shape, most noticeable in larger specimens; the curve of subsequent A-end units decreases towards the mid-point of the body. Units intersect the midline and no offset in units across the midline is observed. The midline lies perpendicular to units at the mid-point of the body. Preservation quality ranges from very good, in which all units are clearly visible, to poor, in which there is very little to no differentiation evident between A- and B-ends. Several specimens contained damaged or indistinct units and were excluded from unit-count data. All specimens are preserved in negative hyporelief and occupy a narrow size range, well below typical maximum size for D. costata (individuals may reach 25 cm in length). Specimens are 6.3–26.7 mm long, with a mean length of 13.28 mm, and are 5.8–22.7 mm wide, with a mean width of 11.86 mm. The major axis regression of (ln) length against (ln) width produced a regression slope of 1.019, with a 95% C.I. of (0.953, 1.089), which is not significantly different from one (Fig. 3.3). If a log-log model is best described by a straight line with slope (the allometric coefficient) equal to one, this suggests isometric growth (Hammer and Harper, Reference Hammer and Harper2006). This appears to be the case in D. costata over the size range examined (Runnegar, Reference Runnegar1982; Evans et al., Reference Evans, Droser and Gehling2017). Comparisons between the linear and several non-linear models using the Akaike Information Criterion (AIC) suggested that a linear model was the best fit for these data (Fig. 3.3; Table 1). Unit number against length was best characterized by models that showed an increase in unit number with size, but at a decreasing rate (particularly for larger specimens). Of those tested, a logistic model best fitted the data based on the AIC (Fig. 3.4; Table 1). This suggests an upper limit on the number of units in D. costata, (Evans et al., Reference Evans, Droser and Gehling2017; Hoekzema et al., Reference Hoekzema, Brasier, Dunn and Liu2017). The size frequency histogram, produced using body length measurements (Fig. 3.1), describes the population distribution of D. costata from the Crisp Wall slabs and supplementary field material as normal (Shapiro-Wilk; W=0.98; p=0.07). A single-component Gaussian finite mixture model best fitted the body length data based on optimization of the BIC, suggesting that these data are best described by a single distribution (rather than multiple distributions).
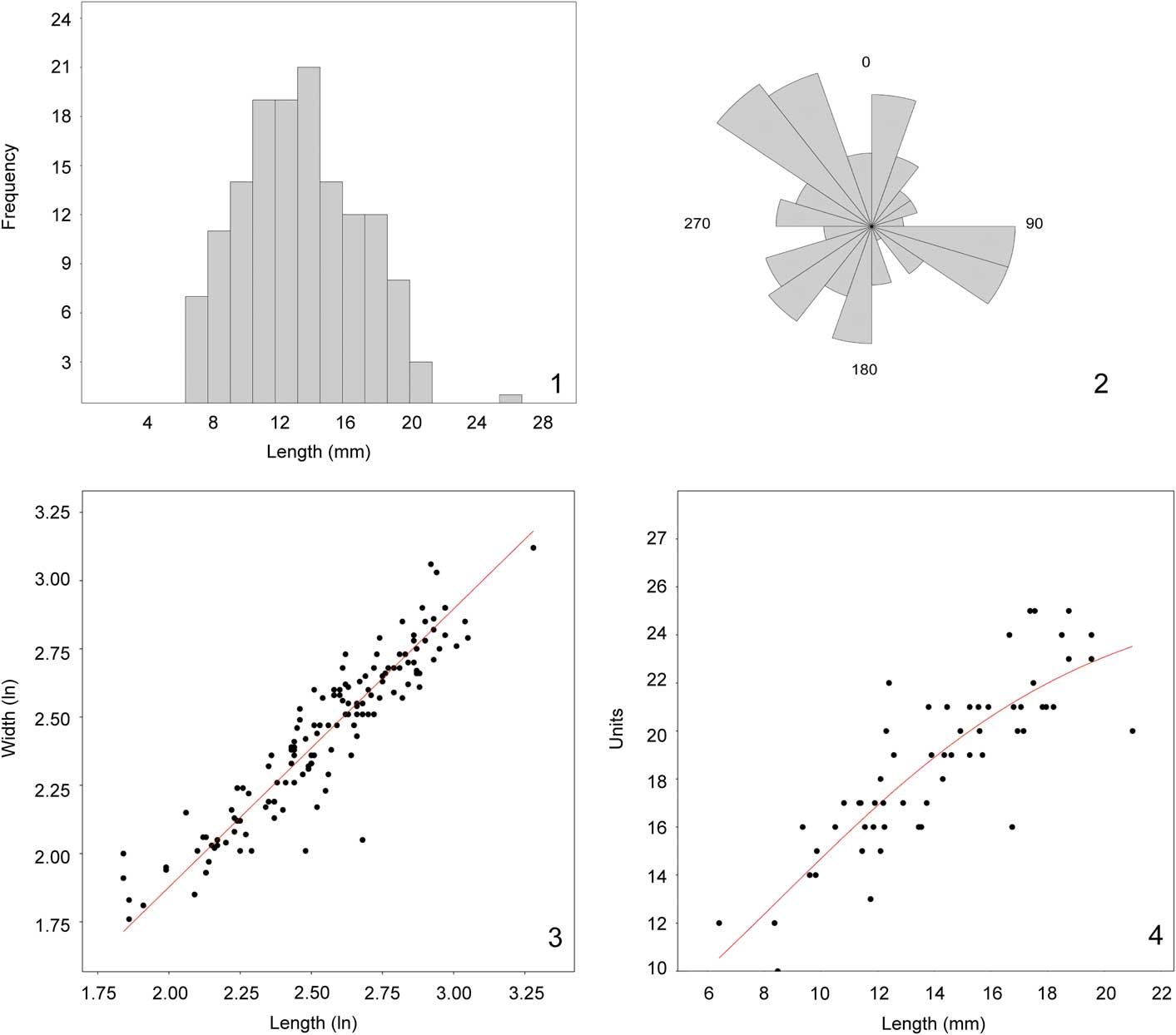
Figure 3 Analyses of Dickinsonia costata population. (1) Histogram showing size distribution of population (N=140); (2) rose diagram showing axial alignment of specimens (N=118); the ‘0’ is an arbitrary reference and denotes the top of the “Crisp Wall” frame; (3) graph depicting the relationship between (ln) length and (ln) width of specimens (N=140); (4) logistic graph depicting the relationship between specimen length and number of observable units (N=58).
Table 1 Akaike information criterion (AIC) values for the body-size variables of Dickinsonia costata from Crisp Gorge, Flinders Ranges. Length=body length, width=body width, units=number of observable body units. The relationship between body (ln) length and (ln) width is best described by a linear function, suggesting isometric growth. Number of body units present against length is best described by a nonlinear, logistic model.

Axial orientation and paleocurrent direction
Based on primary ripple shape and orientation of the bed top surfaces of the Crisp Wall, a primary paleocurrent direction of ~55° from north has been established (Reid et al., Reference Reid, García-Bellido, Payne, Runnegar and Gehling2017). No consistent axial orientation is observed in the Crisp Wall specimens (Fig. 3.2). Hence, there appears to be no relationship between the axial orientation of D. costata and either paleocurrent or burial flow direction.
Morphological features
A marked A-end protuberance occurs in 15% of the Crisp Gorge specimens (Figs. 2, 4). Broadly confined to the terminal A-end unit, this asymmetric bulge appears in deep negative relief relative to the surrounding organism, often developing into a point towards the peripheral margin. Larger protuberances extend for up to 5 mm, in smaller specimens encompassing nearly half the sagittal length. In larger specimens where greater detail is visible, protuberances often observed to be offset from the primary axis, to either the left or the right, towards and sometimes encompassing A-end units. This feature is observed in five other small Dickinsonia within the South Australian Museum collections. The deep relief and consistent positioning of the protuberance exclude the possibility that it is the result of the organism overlying a TOS feature within the substrate.

Figure 4 Photographs of Dickinsonia costata Sprigg Reference Sprigg1947 from the Crisp Gorge fossil locality, Flinders Ranges (latex rubber casts). (1) Well-defined, non-continuous shrinkage rim and A-end lip, SAMP34235; (2) well-developed A-end lip present as narrow, high relief band in A-end units, SAMP34228; (3) continuous shrinkage rim visible around entirety of body, SAMP34243; (4) A-end lip visible on right side of body distorting perimeter of A-end units, SAMP32351; (5, 6) displaying continuous, uneven shrinkage rims SAMP34224, SAMP34219; (7, 8) A-end protuberance present as a bold, high relief structure encompassing terminal A-end units and involving subsequent A-end units, SAMPP34232, SAMP34254; (9) A-end protuberance and shrinkage rim, SAMP34326. Scale bars=5mm.
The A-end lip appears as a raised, upper-surface rim in nearly half (45%) of observed specimens (Figs. 2, 4). It is visible as a rim that outlines the body through the outer margin of the units at a thickness of no more than 1 mm. It occurs most commonly through the A-end and mid-body units, and is visible on some specimens distorting the peripheral margins of individual units.
A ‘shrinkage rim’ feature occurs in 38% of Crisp Gorge specimens, appearing as a positive relief rim extending 1–2 mm from the peripheral margin and in contrast to the negative hyporelief of the body mold (Figs. 2, 4). In most specimens, it does not extend the full circumference of the body. In some instances, however, a pronounced continual peripheral rim surrounds the body and can contain a secondary outline, interpreted as representing a second stage of shrinkage (Gehling et al., Reference Gehling, Droser, Jensen and Runnegar2005). In several specimens where shrinkage is most marked, a faint impression of lower-surface units is visible within the shrinkage rim. A test of correlations between these two features shows a weak, but significant, positive relationship between the occurrence of a shrinkage rim and an A-end lip (Pearson’s correlation coefficient=0.22, p-value <0.01).
Discussion
The abundance and narrow size distribution of the juvenile population on the Crisp Wall surface suggest that Dickinsonia costata may have been an opportunistic species that established on this surface shortly after deposition and stabilization of the underlying substrate (Reid et al., Reference Reid, García-Bellido, Payne, Runnegar and Gehling2017). Dickinsonia is noted throughout all of the fossiliferous facies of the Ediacara Member in a broad range of sedimentary environments and at a range of localities, although more commonly in adult size ranges and usually at lower abundances (Droser and Gehling, Reference Droser and Gehling2015; Evans, Reference Evans2015) than those observed in the Crisp Wall. At the Nilpena fossil locality on the western border of the Flinders Ranges, Dickinsonia is the third most abundant body fossil, exceeded only by Funisia and Aspidella, repectively, both of which represent anchored taxa (Gehling and Droser, Reference Gehling and Droser2013).
Opportunistic species display a range of identifying characteristics, many of which are demonstrated by Dickinsonia. They are typically generalists, capable of survival within a broad range of environmental and physiological parameters, as demonstrated by the identification of Dickinsonia in all fossiliferous facies of the Ediacara Member, and in situ within the majority of these paleoenvironments (Gehling and Droser, Reference Gehling and Droser2013). Species utilizing such a life history strategy may display a rapid increase in population numbers, often evidenced by the numerical domination of a given community or the rapid colonization of substrate previously devoid of organisms (Levinton, Reference Levinton1970). Dickinsonia comprises >50% of the Crisp Wall community, a trait that is not uncommon in other Ediacara Member communities (Droser and Gehling, Reference Droser and Gehling2015).
The Crisp Wall population displays minimal variance in size and is normally distributed, in contrast with other observed D. costata populations. Broadly speaking, Dickinsonia records a right-skewed population and a spread of body sizes spanning juvenile to adult (Zakrevskaya, Reference Zakrevskaya2014; Evans, Reference Evans2015). There are two potential reasons for this discrepancy: (1) this population was the result of a single, isolated reproductive event; and (2) this population was buried in the very early stages of continuous or recurring, episodic recruitment, but was too immature to record a bimodal, clustered, or skewed distribution. Regardless, the distribution of this juvenile population does not negate the likelihood of longer-term continuous recruitment within the taxon, and supports the notion that the Crisp Wall surface records an immature, pioneer-stage community (Reid et al., Reference Reid, García-Bellido, Payne, Runnegar and Gehling2017).
Based on the length to width ratio, Dickinsonia costata displays a juvenile isometric growth pattern (Fig. 3.3). This is consistent with previous findings, which have focused on larger individuals of the taxon and suggest that overall growth was isometric, with length and width increasing at a steady ratio throughout the majority of the organism’s lifecycle (Runnegar, Reference Runnegar1982; Evans et al., Reference Evans, Droser and Gehling2017). This is despite the observed decrease in unit addition, indicating that overall shape is maintained independent of unit number (Evans et al., Reference Evans, Droser and Gehling2017; Hoekzema et al., Reference Hoekzema, Brasier, Dunn and Liu2017). Although all specimens are broadly ovoid in shape, variation in shape does occur. This may be accounted for by flexibility of the organism, and may be related to flexure resulting from movement of the organism in relation to the underlying microbial substrate. It may also be due to natural variation within the population. Likewise, a minor amount of size variation may be accounted for by the presence of the shrinkage rim in organisms that display this feature.
The lack of axial orientation noted in Dickinsonia both in this study and previously is in contrast to observations made for other Ediacaran forms (see Evans et al., Reference Evans, Droser and Gehling2015). Several Ediacaran taxa, including Thectardis avalonensis Clapham et al., Reference Clapham, Narbonne, Gehling, Greentree and Anderson2004 and fronds, including Charnia Ford, Reference Ford1958 have been demonstrated to have been manipulated by the unidirectional current flow at the time of their burial, effectively recording current direction of the burial flow with their final resting position (Wood et al., Reference Wood, Dalrymple, Narbonne, Gehling and Clapham2003; Clapham et al., Reference Clapham, Narbonne, Gehling, Greentree and Anderson2004; Wilby et al., Reference Wilby, Carney and Howe2011). Recent evidence of rheotaxis in Parvancorina suggests that an active response to current flow was employed, with individuals demonstrated to align with the current within two different paleoenvironments, most likely to minimize drag and ensure that current flow was directed over feeding structures (Paterson et al., Reference Paterson, Gehling, Droser and Bicknell2017; Darroch et al., Reference Darroch, Rahman, Gibson, Racicot and Laflamme2017). The absence of current alignment in Dickinsonia may be due in part to its inferred life habit in near-constant contact with the underlying microbial substrate on which it likely fed (Sperling and Vinther, Reference Sperling and Vinther2010). This close relationship with the dense, often ropey, substrate may have made Dickinsonia more or less impervious to current action, with the exception of higher-energy events lifting a small portion of the body away from the substrate (Evans et al., Reference Evans, Droser and Gehling2015).
The correlation between the shrinkage rim and A-end lip suggests that these features may have represented a physical response by the organism to the burial process. The shrinkage rim is also observed in Dickinsonia specimens from a number of localities across a range of sizes and has been interpreted as representing the shrinkage of the organism, due to a loss of mass associated with desiccation during early diagenesis (Gehling et al., Reference Gehling, Droser, Jensen and Runnegar2005). Its correlation with the A-end lip suggests that this feature may also be due to the burial process, and that these features combined could be the result of a muscle-like contraction in response to the burial event, indicating displacement rather than loss of mass, and resulting in a thickening of the peripheral margin of the organism. The occurrence of this lip most commonly through the terminal A-end unit may be due to this unit representing the thinnest section through the fossil, and therefore making it most apparent in this location.
In contrast to these features, the striking A-end protuberance noted in this population is observed in only a limited number of small or juvenile Dickinsonia elsewhere in the South Australian Museum collections. Although broadly limited to the terminal A-end unit, placement of the protuberance within this region appears to be random. The deep-relief nature of the feature suggests that it was largely impervious to the burial process and was therefore likely constructed of the same material as the rest of the organism. Why it appears in such a high concentration in the Crisp Gorge material, but remains relatively rare in other Dickinsonia populations such as those at the Nilpena fossil locality, remains unclear. Given the juvenile size range of this population, however, it appears most likely that this feature is an artifact of early growth and may reflect a biological structure found exclusively in juveniles. Were it to be observed in a single individual, it may be dismissed as a preservational abnormality. It is unlikely, however, that this structure is taphonomic because there is nothing to suggest that the mode of preservation varied based on the size of the organism preserved.
Conclusions
The Dickinsonia costata population from Crisp Gorge provides an excellent opportunity to examine this taxon in juvenile form, and investigate the physical characteristics of such a population. Dickinsonia was likely a hardy opportunist with a single, juvenile size mode, which grew isometrically by maintaining its overall size ratio. The presence of the morphological features observed, including the shrinkage rim and A-end lip, requires further investigation in adult specimens, with the study of larger individuals perhaps providing higher-resolution material for examination. The A-end protuberance described in this population is most likely an artifact of early growth, however, further examination of juvenile Dickinsonia from other Ediacara surfaces will be helpful to confirm this.
Acknowledgments
This research has been supported by a student research grant from the University of Adelaide 2014-007, a summer research scholarship from the University of South Australia PD134814, an Australian Government Research Training Program Scholarship, and a South Australian Museum Early Career Researcher Postgraduate Scholarship to LMR. DCGB is supported by Australian Research Council Future Fellowship FT130101329. Collection and transport of the majority of material utilized in this project was funded by an NSF grant (EAR 9004601) to B.N. Runnegar. We thank C. Reschke and family for access to the Crisp Gorge locality. A. Liu and J. Holmes are thanked for field assistance and feedback. This manuscript benefited from feedback provided by M. Lee, J. Antcliffe, M. Laflamme, and J. Hoyal Cuthill.
Appendix
Physical characteristics and orientation of Dickinsonia costata on Crisp Gorge “wall” surface. 1Axial Orientation is measured relative to a north proxy (top horizontal bar of the Crisp Wall frame). Axial orientation was not measured for specimens in which orientation was indistinct. 2Width and/or length measurements are not included for specimens that were damaged or the outline of the fossil was unclear.








