In the last decades, the global consumption of animal proteins has increased continuously and is expected to increase further in the coming years. At this moment, animal-derived protein accounts for about 60 % of humans’ total protein consumption in developed countries(Reference Pasiakos, Agarwal and Lieberman1). The increasing standards of living in developing countries and the rapid population growth are the main factors responsible for a higher demand of animal-derived protein(Reference Aiking2–Reference Gilland4).
The current production of animal-derived protein, even after intensification, would not suffice to keep up with population growth and the associated requirements for amino acids(Reference Gilland4). Therefore, a transition towards diets containing more plant-derived protein is needed. This transition will also provide benefits for the environment, animal welfare and human health(Reference Aiking2), as a recent study showed that replacing animal protein with plant protein reduced type 2 diabetes risk(Reference Virtanen, Koskinen and Voutilainen5).
To make this shift in diet possible, new plant protein sources have been explored as alternatives, such as (micro)algae, seaweeds, rapeseed and duckweed(Reference Van der Peet and Kamp6,Reference van der Spiegel, Noordam and van der Fels-Klerx7) . Duckweed has attracted considerable attention for several reasons. First, duckweed has a very high growth rate resulting in a high biomass yield per hectare and can tolerate extreme circumstances(Reference Goopy and Murray8–Reference Leng, Stambolie and Bell11). Second, it can be cultivated outdoors in a basin, in (simple) greenhouses, or in multilayer vertical farming systems, thereby not making use of farming land(Reference Leng, Stambolie and Bell11). Third, duckweed contains high amounts of protein (35–43 %) when grown under optimal temperature, light and nutrient availability conditions and harvested regularly(Reference Goopy and Murray8,Reference Leng, Stambolie and Bell11,Reference Appenroth, Sree and Böhm12) . Fourth, duckweed is rich in minerals, vitamins, carotenoids and specific flavonoids and has a favourable fatty acid composition(Reference Appenroth, Sree and Böhm12–Reference Mwale and Gwaze14). Finally, duckweed protein has a better array of essential amino acids (EAA) than most vegetable proteins and more closely resembles animal protein(Reference Iqbal10,Reference Leng, Stambolie and Bell11) . Duckweed is also known for its remediation capacity, as metals and other pollutants can be absorbed by the plants. However, this is often in low quantities and foremost caused by growing on contaminated water(Reference van der Spiegel, Noordam and van der Fels-Klerx7,Reference Appenroth, Sree and Böhm12,Reference Leng15) .
Duckweed has long been used as animal feed. Fresh and dried duckweed has been fed successfully to fish, waterfowl, chickens, cattle, pigs, sheep and goats with good results on growth performance when duckweed was part of their habitual diet(Reference Goopy and Murray8,Reference Mwale and Gwaze14,Reference Tavares, Rodrigues and Fracalossi16) , indicating that duckweed can be part of the diet without adverse effects.
Furthermore, duckweed has been eaten by humans for generations as a nutritious vegetable – named ‘Khai-Nam’ – in Southeast Asian countries, including Laos, Thailand and Burma (now Myanmar)(Reference Bhanthumnavin and McGarry17). In these countries, Wolffia arrhiza and W. globosa are the dominating species used for human consumption(Reference Appenroth, Sree and Böhm12,Reference Sree and Appenroth18) . So far, no reports of adverse effects of eating duckweed by humans are known(Reference Appenroth and Sree19).
Before implementing duckweed in the human diet, it is important to know the nutritional value in comparison with other plant-based protein sources. However, hardly any information is available on the digestion and nutritional value of proteins from whole vegetables in humans. Most studies on nutritional values have been performed based on isolated protein. The literature has shown that digestibility of these isolated protein in humans can vary between different sources(Reference Bos, Metges and Gaudichon20,Reference Farnfield, Trenerry and Carey21) , which affects amino acid availability in the blood. Digestion values of duckweed protein in animals have been reported to vary between 50 and 88 %(Reference Damry, Nolan and Bell22,Reference Du Thanh, Nguyen Quang and Everts23) , but no data are available for humans. In addition, different protein sources may also lead to different postprandial glucose and insulin responses(Reference Comerford and Pasin24,Reference Crowder, Neumann and Baum25) .
Because information about duckweed digestibility in humans is lacking, the primary objective of the present study was to assess the postprandial serum amino acid profile after a single intake of duckweed in healthy adult volunteers in comparison with another high-protein plant source: green peas. Secondary objectives were to assess postprandial plasma glucose and insulin responses and to assess the acute health parameters heart rate, blood pressure and aural temperature and the occurrence of gastrointestinal complaints as part of the safety assessment in humans. The present study was conducted by using Lemna minor that showed good growth characteristics when grown indoor in our facilities.
Methods and materials
Subjects
Subjects were recruited via the pool of volunteers of Wageningen Food and Biobased Research. Inclusion criteria were: aged between 18 and 50 years, healthy as assessed by a health and lifestyle questionnaire, normal blood pressure, normal blood clinical laboratory tests for Hb, kidney and liver functioning (alanine aminotransferase (ALAT), γ-glutamyl transferase (GGT), creatinine and estimated glomerular filtration rate (eGFR)), appropriate veins for blood sampling and a BMI between 19 and 25 kg/m2. Subjects were excluded when they had any metabolic, gastrointestinal, inflammatory or chronic disease (such as diabetes, anaemia, hepatitis, CVD), a history of gastrointestinal surgery, liver dysfunction (cirrhosis, hepatitis), liver surgery or kidney dysfunction (eGFR <60 ml/min). Other exclusion criteria were: use of gastric acid inhibitors or laxatives, use of hard drugs, taking three or more glasses of alcoholic beverages per d, being pregnant or lactating, participating in intense sporting (>16 h per week) and being a current smoker. Subjects who had known allergies towards the products used in the study, or were not willing to consume chicken broth, were also excluded.
A sample size calculation for paired comparisons with P = 0·05 (two-sided, Zα = 1·96), Zβ = 1·28 (power of 90 %), an individual difference in plasma peak values of 100 µg/ml (=sd)(Reference Farnfield, Trenerry and Carey21,Reference He, Spelbrink and Witteman26) and a difference of 100 µg/ml regarded as interesting (=δ) indicated that eleven subjects would be sufficient. To account for potential dropout, fifteen subjects were included in the study. Of the participants, three dropped out of the study, because they could not finish the L. minor product within the given time frame of 20 min. Therefore, the final sample consisted of twelve participants. Fig. 1 shows the flow of the study participants.

Fig. 1. Flow of participants through the randomised trial.
Study design and procedures
The trial was carried out in November 2017. Participants were instructed to avoid alcohol and heavy physical activity for 24 h before the test day, and to maintain their habitual dietary pattern. They received a ready-to-eat pasta meal for the evening before the test day and were instructed to consume this between 18.00 and 20.00 hours (no dessert). Subsequently, participants fasted after this meal (only water or tea allowed without sugar and without milk).
On arrival at the research facilities in Wageningen, The Netherlands, heart rate and blood pressure were measured with an Omron HEM 907 via standardised procedures, as well as aural temperature (to the nearest 0·1 degree). Subsequently, a cannula was inserted for blood sampling. After a baseline blood sample, subjects received the test product warm together with 100 ml water. The L. minor product had the appearance of a heated portioned frozen spinach, whereas the pea product looked like a bound soup. Subjects were instructed to finish the product within 20 min and spread consumption evenly over this time period. Blood samples were taken at 15, 30, 45, 60, 75, 90, 120, 150 and 180 min after completion. Participants completed a short sensory questionnaire to evaluate the test products. The attributes green colour, intensity of smell, pleasantness of taste, thickness and bitterness of the product were evaluated on a seven-point scale from 1 = not at all to 7 = very much. At the end of the 3 h, heart rate, blood pressure and aural temperature were measured again. In a diary, subjects recorded any gastrointestinal complaints for the test day and the following 3 d. At 1 week later, the same procedures were repeated, but with the other protein source.
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Medical Ethical Committee of Wageningen University (protocol no. 17/13). Written informed consent was obtained from all subjects. The study was registered in The Netherlands National Trial Register, reference no. NTR6516 (http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=6516).
Products
For the present study, L. minor (accession 8623) was used. This accession line was obtained in 2011 from a stock culture maintained at the laboratory of Professor Dr Klaus Appenroth (Friedrich Schiller University, Jena), and cultivated and bio-banked at Wageningen Plant Research, Bioscience laboratory from that time on. L. minor (8623) plants starting from sterile cultivation were grown in 40-litre containers in an indoor growth chamber on a defined medium (CuSO4.5H2O, 0·0838 mg/l; H3BO, 2·896 mg/l; MnSO4.1H2O, 2·145 mg/l; Na2MoO4.2H2O, 0·135 mg/l; ZnSO4.7H2O, 0·536 mg/l; CaCl2.2H2O, 64·35 mg/l; KH2PO4, 103·25 mg/l; KNO3, 394·25 mg/l; MgSO4.7H2O, 132·25 mg/l; Fe-EDDHA (ethylenediamine-N,N′-bis(2-hydroxyphenylacetic acid), 20·45 mg/l). Water was aerated and recycled via UV filters to suppress contaminations in the water. Plants were kept under a day length of 16 h, and day and night temperatures of 23 and 20°C, respectively, a photon flux density of 300–325 µmol/m2 per s, a CO2 concentration of approximately 400 parts per million and a relative humidity of 75 %. Approximately 40 % of the growth surface were harvested weekly, washed, freeze dried and stored at −20°C. The final batch of L. minor was analysed in the laboratory for nutritional, microbiological and toxicological content (Nutrilab Giessen; Ansynth Service BV Roosendaal, Wageningen Plant Research and RIKILT-Wageningen). Based on these results, no harm was expected from a single intake in humans. Nutritional content and toxicological measures are shown in the results. The reference product was commercially available green peas (frozen green peas; Bonduelle), which were bought in a local supermarket. Both products were freeze dried and stored in the freezer until use.
The test products were prepared in a research kitchen at the morning of each test day. For an equivalent of 20 g of protein, 64 g of freeze-dried L. minor or 84 g freeze dried green peas were mixed with water, chicken broth and onion (including totally 4 g butter, 30 g of fresh cut onion, one tablet chicken broth: Maggi; Nestlé) per person portion. The products were cooked for 10 min at 100°C and then served. One portion was 550 g of test product. The test products were coded with a two-digit number and were offered in random order to the participants, on condition that at least two subjects per test day had a similar product. This allocation of treatment to participants was done by a researcher who was not involved in the study. Blinding of the products was not possible, due to an obvious difference in appearance.
Measurements
The blood samples were stored at −80°C until further analysis. Plasma insulin and glucose levels were assessed in the Gelderse Vallei Hospital in Ede, using standardised clinical procedures. Plasma amino acids were determined at Ansynth Service (Roosendaal) according to the procedure of Terrlink et al.(Reference Terrlink, van Leeuwen and Houdijk27). Plasma amino acids were analysed by HPLC after precipitating plasma proteins in 50 µl plasma with 200 µl 3 % perchloric acid. Individual amino acids were determined by ultra-fast liquid chromatography (UFLC, an HPLC of Shimadzu) using a pre-column derivatisation with o-phthaldialdehyde and fluorimetric detection.
The following gastrointestinal symptoms were included in the participant diary: bloated feeling, belching, flatulence, nausea, abdominal pain, diarrhoea, constipation(Reference Bovenschen, Janssen and van Oijen28–Reference Veenstra, Duncan and Cryne31). On the test day and the subsequent 3 d, participants scored these symptoms on a seven-point scale with the anchors 1 = not at all present to 7 = strongly present. Adverse events were registered by self-reporting and questioning by a medical doctor. Adverse events were classified under the responsibility of the medical doctor according to 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) coding.
Statistical analysis
Statistical analyses were done using IBM SPSS statistics 23 with a P value of P < 0·05 considered as statistically significant. Participant characteristics are shown as means with their standard errors (age, BMI) or as percentages (sex).
Blood plasma values that were below the detection threshold were replaced by the half-value of the detection threshold (this was done for the amino acids asparagine acid, hydroxyproline and cysteine, as well as for insulin where <2·0 mU/l was recoded as 1·0). The plasma values of the individual amino acids were summed up to calculate the total amino acids (TAA) and EAA.
For the blood plasma amino acids (TAA, EAA), glucose and insulin, a linear mixed-model analysis for repeated measures with subject as random factor and time (ten levels) and protein source (two levels: L. minor + peas) as fixed factors was used to assess main effects of time and protein source, as well as an interaction effect time × protein source. In the case of a significant interaction between time and protein source, post hoc tests were applied to locate the differences between the two protein sources at each time point with Bonferroni correction (differences were considered significant when P < 0·005, in order to correct for multiple comparisons).
In addition, TAA, EAA, glucose and insulin peak values and time to peak were calculated for both protein sources (means and standard errors). A paired t test (within-subject design) was used to assess significant differences between the two protein sources in peak value and time to peak.
For aural temperature, blood pressure and heart rate, mean values with their standard errors were calculated per condition (peas v. L. minor) and per time point (t = 0 and t = 180). A mixed model with time and protein source as fixed factors and subject as random factor was used to determine main effects of time and protein source as well as the interaction effect time × protein source.
To get insight into the occurrence of gastrointestinal complaints, the scores given by the respondents on the seven-point scale were recoded. A score of five or higher was coded as ‘complaint present’ whereas scores below five were recoded as ‘complaint not present’. Subsequently, the number of gastrointestinal complaints were counted over the 4 d and cross-tabulated per test product. In addition, individual scores per gastrointestinal complaint were averaged across the 4 d per participant. Subsequently, mean scores with their standard errors were calculated per condition and tested with a paired t test. Also for the sensory attributes, mean scores with their standard errors were calculated for L. minor and peas and were compared with a paired t test.
Results
Macronutrient content
Table 1 shows the general nutrition content, amino acid composition and metal concentrations of L. minor and green peas in their freeze-dried form. As a comparison, the nutritional composition of egg was added as an example of an animal source. Protein content in L. minor (31·3 %) is higher than in peas (23·8 %), whereas sugar content is higher in green peas (18·9 %) than in L. minor (1·2 %). Fibre, starch and fat content are relatively similar in the two products. Egg is fully composed of protein and fat, and only a minor concentration of sugar, and does not contain fibres or starch.
Table 1. General nutrition content and metal concentrations of freeze-dried Lemna minor and green peas

E, essential; N.D., non-detectable.
* NDB no. 01129; US Department of Agriculture National Nutrient Database for Standard Reference, Legacy. Current version: April 2018 (http://www.ars.usda.gov/nutrientdata).
† Mono- and disaccharides and reducing sugars conform to the methods NEN 3571 and EU152/2009.
‡ Phenylalanine + tyrosine.
§ Cd, Hg and Pb values were below the detection limit.
The products show a comparable amino acid profile for almost all amino acids and the EAA levels are very close or higher compared with the advised WHO levels(32). The only exception to this is the non-EAA aspartic acid + asparagine, of which the level in L. minor is relatively high.
In general, metal concentrations in L. minor were lower than the safe limits of intake, except for As levels which were higher than threshold levels set by the food authorities for rice(33), but of little concern for a single intake.
In some publications, calcium oxalate has been mentioned as a potential risk factor for large-scale and long-term intake in humans, as this component might reduce Ca absorption in the intestine or can induce kidney stones(Reference Champ34,Reference Noonan and Savage35) . The total calcium oxalate content in our batch of L. minor (209 mg/g freeze-dried duckweed) was much less than in spinach (which is 4·4× higher than L. minor) and rhubarb (3× higher than L. minor).
Subjects
The twelve subjects were on average 31·7 (sem 3·04) years old, had a BMI of 22·7 (sem 0·38) kg/m2 and 42 % were female (n 5).
Plasma amino acid profile after consumption of the two protein products
Figs 2 and 3 show the profile for serum concentrations of TAA and EAA after consumption of L. minor or peas. Baseline values (T0) were not significantly different between L. minor and peas for TAA (P = 0·82) and EAA (P = 0·88). For peas, TAA rose sharply after ingestion and decreased again after 45 min, whereas for L. minor, TAA increased only slightly and remained relatively stable. The figures obviously show that the AUC is lower for L. minor compared with green peas, indicating that L. minor protein is not well digested and absorbed.
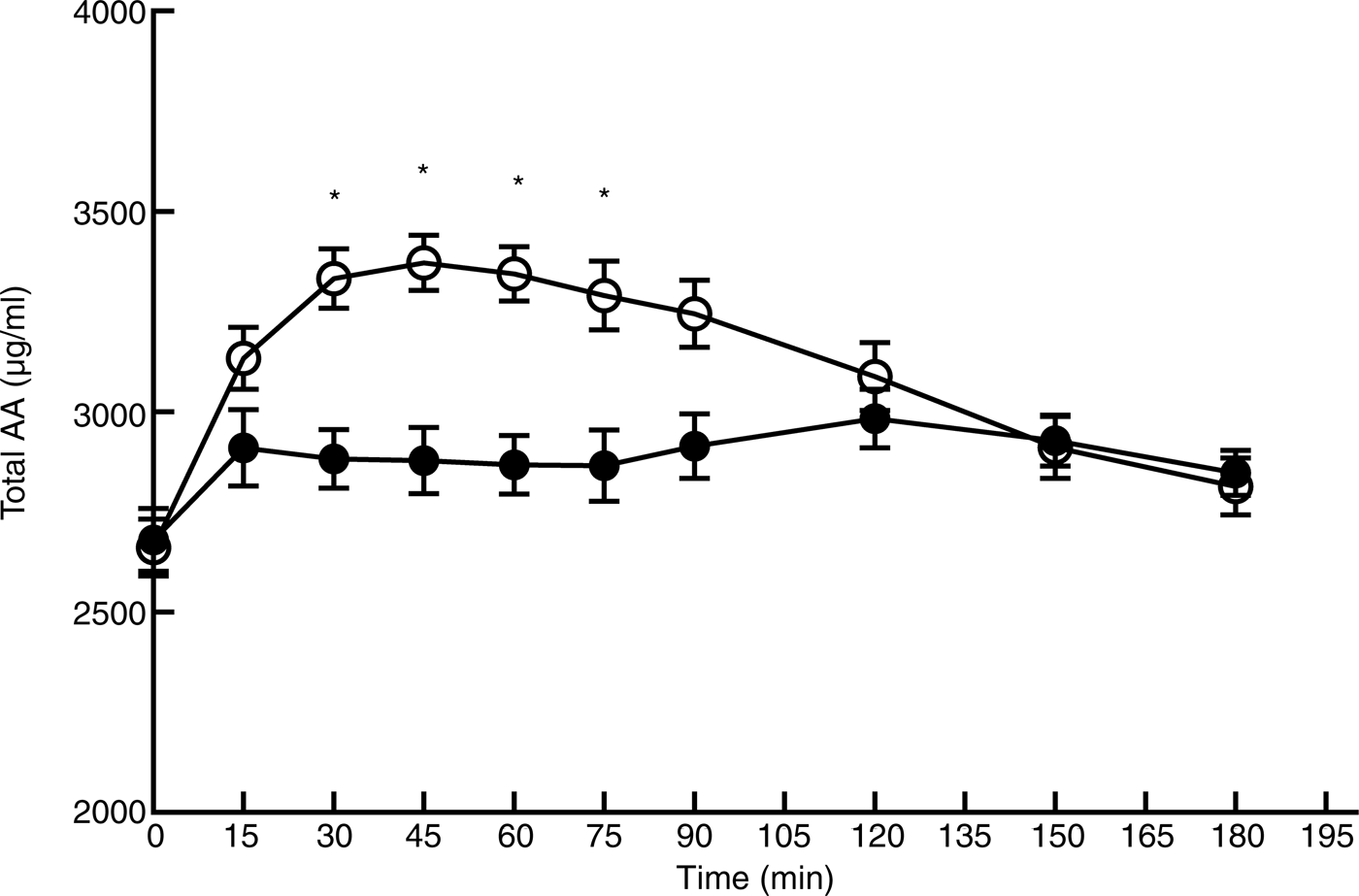
Fig. 2. Plasma amino acid profile for total amino acids (AA) after consumption of Lemna minor (–●–) and green peas (–○–). Values are means, with standard errors represented by vertical bars. * Significant difference at P < 0·005 (post hoc t test).

Fig. 3. Plasma amino acid profile for essential amino acids (EAA) after consumption of Lemna minor (–●–) and green peas (–○–). Values are means, with standard errors represented by vertical bars. * Significant difference at P < 0·005 (post hoc t test).
The mixed-model analysis for TAA showed a significant main effect of time (F (9,206) = 14·76; P < 0·0001), treatment (F (1,206) = 102·70; P < 0·0001) and interaction (F (9,206) = 8·18; P < 0·0001). Pairwise comparisons showed significant differences between L. minor and peas at four time points. A similar picture was seen for EAA, with a significant main effect of time (F (9,206) = 14·87; P < 0·0001), treatment (F (1,206) = 309·44; P < 0·0001) and interaction (F (9,206) = 17·28; P < 0·0001). EAA levels for L. minor and peas were significantly different at six time points (see Fig. 3).
Peak values and time to peak for TAA and EAA are shown in Table 2. Peak values for EAA and TAA were significantly higher for peas compared with L. minor, whereas time to peak was approximately twice as high for L. minor for both EAA and TAA. These results confirm the figures, indicating that L. minor protein appears in the blood later and with a lower peak.
Table 2. Peak values and time to peak for total amino acids (TAA), essential amino acids (EAA), glucose and insulin after ingestion of Lemna minor and green peas
(Mean values with their standard errors; n 12)
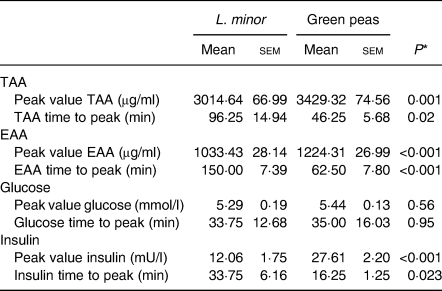
* By t test.
For both L. minor and green peas, the first limiting amino acid based on the peak value of the postprandial amino acid levels was calculated to be methionine. This was especially true for L. minor, where methionine was 15× lower than the WHO recommendation, whereas for peas, methionine was five times lower.
Plasma glucose and insulin profile after ingestion of the two protein products
The baseline values (T0) for glucose and insulin were not significantly different between L. minor and peas (P = 0·75 and P = 0·74, respectively). For glucose (Fig. 4), there was a significant main effect of time (F (9,206) = 10·03; P < 0·0001), treatment (F (1,206) = 28·12; P < 0·0001) and interaction (F (9,206) = 5·56; P < 0·0001). Whereas glucose levels remained relatively stable after L. minor consumption, glucose peaks at t = 15 after consumption of peas and then drops quickly until t = 45, after which glucose levels stabilise again. Also for insulin (Fig. 5), there was a significant main effect of time (F (9,206) = 68·52; P < 0·0001), treatment (F (1,206) = 124·23; P < 0·0001) and interaction (F (9,206) = 21·53; P < 0·0001). Again, insulin levels remain relatively stable after ingestion of L. minor, but sharply peak after ingestion of green peas. These results indicate that the postprandial glucose and insulin responses are different after ingestion of L. minor and pea products.

Fig. 4. Plasma glucose profile after consumption of Lemna minor (–●–) and green peas (–○–). Values are means, with standard errors represented by vertical bars. * Significant difference at P < 0·005 (post hoc t test).

Fig. 5. Plasma insulin profile after consumption of Lemna minor (–●–) and green peas (–○–). Values are means, with standard errors represented by vertical bars. * Significant difference at P < 0·005 (post hoc t test).
There were no significant differences between L. minor and peas for the peak values of blood glucose and the time to peak for glucose (see Table 2). Peak values of insulin were higher for peas (P < 0·001) compared with L. minor and time to peak for insulin was higher for L. minor (P = 0·02). So, after ingestion of L. minor, insulin levels peak lower and later than after ingestion of peas.
Blood pressure, heart rate and temperature
Table 3 shows the mean values for blood pressure, heart rate and aural temperature before and after consumption of the two products. A mixed model with time (T0 and T180) and protein type (peas and L. minor) as fixed factors and subject as random factor showed no significant interaction effect (F (1,33) = 0·02; P = 0·90). The main effects were also non-significant (time: F (1,33) = 0·21, P = 0·65; protein type: F (1,34) = 1·22, P = 0·28). So, there were no significant differences in these health parameters between the two time points or between the two protein-rich products.
Table 3. Blood pressure, heart rate and aural temperature before (T0) and at 3 h (T180) after consumption of the two protein products
(Mean values with their standard errors; n 12)

bpm, Beats per min.
Gastrointestinal symptoms
No serious adverse events were observed during the study. The occurrence of gastrointestinal symptoms over the 4 d after consumption was relatively low, with no clinically relevant differences in occurrence between the two products.
After consuming the peas, six complaints were reported by two subjects (5 × flatulence and 1 × diarrhoea). After consuming L. minor, ten complaints were reported by four subjects (2 × belching, 1 × flatulence, 2 × nausea, 4 × abdominal pain and 1 × diarrhoea).
Table 4 confirms the low incidence of gastrointestinal symptoms. For both products, average scores per symptom were all below a score of two on a seven-point scale, with no significant differences between L. minor and peas (all P > 0·12).
Table 4. Average scores* for the gastrointestinal symptoms that were reported by the participants in the 4 d after consuming the two protein products
(Mean values with their standard errors; n 12)

* 1 = Not at all present; 7 = strongly present.
† By t test.
Sensory evaluation
Table 5 shows the mean scores for the sensory attributes of the two protein products. L. minor was significantly less liked (P < 0·001) and evaluated as more bitter (P = 0·003) compared with the green pea product. Surprisingly, despite the apparent difference in texture, thickness was not perceived as significantly different.
Table 5. Sensory characteristics of the Lemna minor and green pea products
(Mean values with their standard errors; n 12)

* By t test.
Discussion
The primary objective of the present study was to analyse the protein uptake from L. minor plant material as human food by the means of postprandial serum amino acid profile after a single intake in healthy adult volunteers in comparison with green peas. To our knowledge, this is the first study that evaluated the postprandial effects of L. minor consumption in humans.
The protein content in our batch of plant material (31·3 %) falls within the range that previous papers stated for Lemna (25–43 %)(Reference Goopy and Murray8,Reference Leng, Stambolie and Bell11,Reference Appenroth, Sree and Böhm12) . Fat content was comparable to the L. minor results of Appenroth et al. (Reference Appenroth, Sree and Böhm12), whereas starch content was much higher in our batch of L. minor (21·4 v. 4 %) which can be caused by differences in environmental conditions, growth medium and/or accession used. The amino acid composition of our product (analysed as full product, not as protein extract) showed some small differences compared with the literature; however, when comparing the EAA, our data give a 0·96 correlation with values for L. minor of both Dewanji(Reference Dewanji36) and Appenroth et al. (Reference Appenroth, Sree and Böhm12). Comparison of the amino acid composition with the amino acid requirements for adults(32) indicates that the concentrations of these EAA in our batch were all slightly higher than the requirements for adults except for methionine (1·6 g/100 g protein WHO v. 1·4 g/100 g protein in L. minor). Overall, it can be concluded that L. minor is an interesting protein source from a nutritional content perspective.
The present study showed large differences between duckweed and peas in the postprandial plasma levels of amino acids. This implies that digestion and absorption of protein from this large bolus of L. minor plant material is relatively low compared with peas. This low digestion may be explained by anti-nutritional factors in the L. minor, such as trypsin inhibitor, calcium oxalate, tannin and phytate(Reference Kalita, Mukhopadhyay and Mukherjee37). Also animal studies indicate that anti-nutritional factors may be present which can limit intake and growth when animals are fed duckweed at high levels(Reference Goopy and Murray8,Reference Solomon and Okomoda38) .
Hardly any publications are available regarding the digestion and uptake of protein from whole food products like vegetables, especially leafy vegetables, based on similar human studies. Most studies assessing postprandial amino acids profiles are based on isolated protein extract which will be quite different from whole products as firmness of cell walls, presence of dietary fibres, anti-nutritional factors, freeze drying and other process or matrix effects can have a large effect on the digestibility and bioavailability of nutrients(Reference Millward, Layman and Tomé39,Reference Morifuji, Ishizaka and Baba40) . This is nicely illustrated by the study of Rutherfurd et al. (Reference Rutherfurd, Fanning and Miller41) in which the protein quality of peas and isolated protein from the same peas were analysed. In the present study, both the PDCAAS (protein digestibility-corrected amino acid scores) and DIAAS (digestible indispensable amino acid scores) as prescribed by the FAO(42) were used to quantify the protein quality. The assessed PDCAAS for cooked peas was 0·597 and the DIAAS 0·579, being much lower than what was found for pea protein concentrate for which a PDCAAS of 0·893 and DIAAS of 0·822 were found, indicating a clear effect of the matrix on digestibility.
The observed differences in amino acid availability for the two plant sources L. minor and peas are only relevant for populations with a marginal protein intake, for whom the consequences of overestimating protein quality may be severe. In general, the Western population eats more protein than is minimally recommended(Reference Van Rossum, Fransen and Verkaik-Klooseterman43,Reference Westhoek, Lesschen and Leip44) and no direct problems can be expected when not being able to release all proteins from a food product consumed. In this respect, it should be noted that the present study does not give insight in the digestion rate of a normal portion size. The 20 g duckweed protein consumed here in one bolus equals 1111 g of fresh L. minor material, a portion size not normally consumed in one meal. Some of the study participants had a hard time finishing the large portion in time. Three participants could not finish the duckweed portion in time and were omitted from the data analysis. Potentially, the firmness of the product and the intense chewing that was required caused some sensory satiation(Reference Hetherington, Havermans and Blundell45) together with a full stomach, resulting in a reluctance to eat further and being unable to finish the portion in time. As bulk size may influence the efficiency of digestion(Reference Dias, Huelvan and Dinis46), it would be interesting to study whether normal portion sizes of L. minor as vegetable have a relatively better digestion and uptake than represented here in the present study.
The different postprandial glucose and insulin responses after ingestion of L. minor and peas in the present study can be explained by the different sugar levels in the raw product. The sugar content of freeze-dried green peas was 18·9 %, whereas the sugar content of L. minor was only 1·2 %. The used portion per person therefore equals 15·9 g sugars for peas and 0·8 g for L. minor. This large difference obviously explains the differences in postprandial glucose and insulin responses, and implies that consumption of L. minor will hardly have any impact on blood insulin levels.
Limitations and strengths
A strength of the present study is that we demonstrated differences in protein digestibility of whole plant products in humans by a straightforward human trial design. Although not as exact as studies based on labelled proteins, the present study indicates clear differences in digestion and uptake when offering a bolus that equals 20 g of protein present in different products. In the present study, we compared the bioavailability of plant protein present in a seed crop (peas) with proteins present in a ‘leaf crop’ (L. minor). The lower bioavailability of Lemna protein compared with peas warrants further research on the digestibility of other leafy vegetables by the same study design. We argue that such studies should be performed systematically for food products (and not only protein isolates) and especially new protein-rich food products, such as those that replace animal-based proteins, in order to gain insight into the true nutritional impact of these products. The measurements of amino acids, glucose, insulin, the various health parameters and gastrointestinal complaints add to obtaining a comprehensive picture, which shows that one very large intake of Lemna plants – grown under controlled conditions – may not lead to high postprandial amino acids levels and was well tolerated by healthy adults.
A limitation of the present study is the fact that blinding of the treatment was not possible because whole food products were tested which are easy recognised by shape, taste and texture. The L. minor and pea products could not be identically matched on sensory attributes, so the participants could recognise both products. Despite an obvious difference between the two products regarding texture, this texture difference was not reported by the respondents in the sensory evaluation. Yet, they reported a lower liking for the L. minor product and a higher score for bitterness. Recognition of the product could have affected the number of gastrointestinal complaints reported as subjects might have been more on guard when consuming an uncommon food product, such as L. minor. However, no significant differences were found between the two protein sources, so it seems likely that these sensory differences did not influence our gastrointestinal results. A second limitation is that we have no information about the amino acid profiles after 180 min and we therefore recommend expanding sampling time when analysing protein products with an expected low digestion and uptake rate.
Implications and recommendations
The proteins in the L. minor plants seem to be less easily available, resulting in a lower digestibility, and a subsequent lower appearance in the blood. This product will be of interest for the protein transition to increase the sustainability of consumer diets, especially in developed countries, where efficient utilisation of proteins due to underweight or protein malnutrition is less of an issue. Because consumers generally respond positively towards trying and consuming duckweed(Reference de Beukelaar, Zeinstra and Mes47), duckweed might have great potential to become a widely accepted food source. However, due to the low protein digestibility of L. minor as a plant, it is recommended to also investigate the digestibility of isolated Lemna protein, because this is probably easier to digest and absorb as shown for other protein extracts and hydrolysates(Reference Morifuji, Ishizaka and Baba40). However, before Lemna plants or proteins can be part of the habitual human diet, a successful Novel Food Application should be completed.
The present study indicates that a single, relatively large bolus of L. minor plants is well tolerated by humans when it is grown under well-controlled circumstances. Nevertheless, Lemna can accumulate heavy metals even when grown on high-quality tap water as available in The Netherlands. The amounts detected in the plant in our batch were not expected to be threatening when taken at a single occasion. Still, in subsequent studies, demi-water or source water and fertilisers with low concentrations of undesired metals and certain minerals should be used to minimise accumulation in the plants. Regularly measuring metal concentrations in the water and harvested plants should be part of the standard quality and safety analysis for L. minor. In earlier studies, calcium oxalate content of duckweed grown under natural conditions in the wild was reported as potentially harmful. Oxalate may hinder the Ca uptake in the body and may increase the risk of kidney stones(Reference Champ34,Reference Noonan and Savage35) . The batch in the present study contained less oxalate than in commercially available spinach and rhubarb, and provided no risk from a single intake. Subsequent studies should investigate the digestion and safety effects of repeated consumption of L. minor.
Conclusion
The results of this human trial showed that proteins from L. minor consumed as whole plant product end up in the blood at lower concentrations compared with green peas, indicating low protein digestibility in humans. Due to the lower sugar content, L. minor had more positive plasma profiles for glucose and insulin compared with peas. There were no significant differences between the two protein-rich products regarding the assessed health parameters or the number of gastrointestinal complaints, providing the first indications that consuming a single meal of L. minor – grown under strict controlled circumstances – is well tolerated by humans.
Acknowledgements
The participants are thanked for their participation in the study. Hetty Busink is thanked for her assistance in the nutritional, microbiological and toxicological analyses. Sandra van der Haar is gratefully acknowledged for contacting and planning the appointments with the participants.
The present study was supported by the Wellcome Trust within the Our Planet, Our Health programme (grant no. 106876/Z15/Z). The funder had no role in the study design, data collection and analyses, or the preparation of the manuscript.
G. G. Z. and J. J. M. designed the study, together with H. F. and I. K.-K.; I. M. M. coordinated the cultivation of Lemna together with J. J. M., whereas I. M. M., D. S. and J. J. M. were involved in harvesting and preparing the product; I. M. M. organised and performed the nutritional, microbiological and toxicological analyses of the L. minor; G. G. Z., D. S., E. O., H. F., I. K.-K. and J. J. M. were all involved in the execution of the trial; G. G. Z. and J. J. M. analysed the data and wrote the manuscript; I. M. M. and J. J. M. coordinated the research project on L. minor as a new plant protein source for human consumption. All authors critically provided feedback on the manuscript.
There are no conflicts of interest.












