Article contents
Yttria-stabilized zirconia crystallization in Al2O3/YSZ multilayers
Published online by Cambridge University Press: 25 January 2012
Abstract
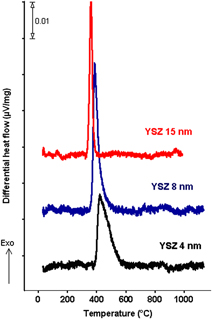
Yttria-stabilized zirconia (YSZ)/Al2O3 multilayers deposited on Pt foil were studied by differential scanning calorimetry. Observed thermal effects were interpreted using additional evidence from x-ray diffraction and transmission electron microscopy. The crystallization temperature of YSZ increases from 344 to 404 °C as the layer thickness decreases from 15 to 4 nm. The enthalpy of crystallization becomes more exothermic with decreasing thickness, and it was measured to be −26 kJ/mol YSZ for 4-nm-thick layers and −12 kJ/mol for 15-nm-thick layers. The latter value is consistent with the reported crystallization enthalpy for YSZ powder of the same composition prepared by precipitation from aqueous solution. The more exothermic crystallization enthalpies for thinner films are indicative of a decrease in their degree of crystallinity. The 2–6-nm-thick Al2O3 layers remain amorphous when heated to 1000 °C. The described methodology enables thermal analysis of oxide thin films using commercial instruments.
Keywords
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2012
References
REFERENCES
- 5
- Cited by


