Article contents
Waterborne acrylic hybrid adhesives based on a methacrylate-functionalized porous clay heterostructure for potential lamination application
Published online by Cambridge University Press: 15 August 2017
Abstract
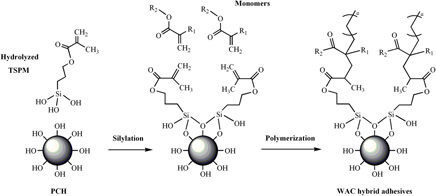
A new waterborne acrylic (WAC) hybrid adhesive was evaluated for an untreated polypropylene lamination. The WAC hybrid adhesive was formulated with a new class of porous clay heterostructure (PCH), which was modified with 3-(trimethoxysilyl)propyl methacrylate (as a coupling agent) to promote chemical bonding with the acrylic matrix to form a methacrylate-functionalized PCH (MPCH). The WAC hybrid adhesive was based on copolymers (2-ethylhexyl acrylate, ethylene glycol methyl ether acrylate, 2-(hydroxyethyl) methacrylate, styrene and acrylic acid) with varying amounts of MPCH. The scanning electron microscopy micrographs revealed the presence of a well dispersed MPCH distributed throughout the matrix. The optimal adhesive performance, in terms of the 180° peel strength of bonded joints, of 140.2 N/m was achieved using 1.5 wt% of MPCH, while the thermal stability of the adhesives was improved with increasing MPCH loading levels.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2017
Footnotes
Contributing Editor: Sarah Morgan
References
REFERENCES
- 2
- Cited by



