Article contents
Three-dimensional anodes prepared by electrodeposition of β-PbO2 onto reticulated vitreous carbon with different porosities
Published online by Cambridge University Press: 03 October 2016
Abstract
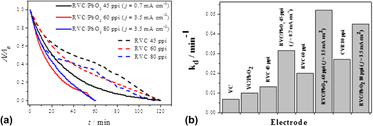
The aim of this study was to obtain a tridimensional electrode with an even greater specific surface area (a s), using the electrodeposition of PbO2 films onto 45, 60, and 80 ppi RVC substrates applying different current densities. The kinetics and efficiency of decolorization of Reactive Blue 19 (RB19) dye achieved with the 60 ppi RVC/PbO2 were higher than obtained with a flat PbO2 electrode and 45 ppi RVC/PbO2, due not only to a greater electrochemically active area, but also especially due to the substantial increase in mass transfer caused by the turbulence generated by the porous matrix. It was found that the coating and the formation of β-PbO2 was only possible on the 60 ppi substrate applying 3.5 mA/cm2; in the case of the 80 ppi substrate, the uneven distribution of potential and current within the porous electrode did not allow establishment of suitable operational conditions that permitted complete and uniform coating of the substrate.
Keywords
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2016
References
REFERENCES
- 7
- Cited by



