Published online by Cambridge University Press: 15 February 2019
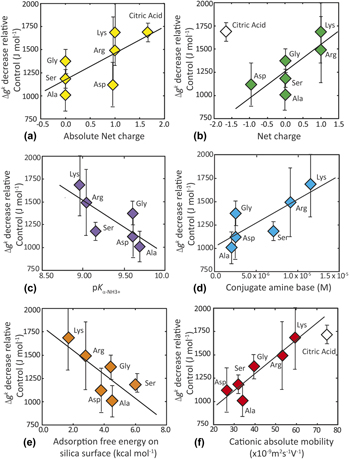
The kinetics of silica polymerization was measured in silicic acid solutions containing a suite of 0.1 M amino acids, 0.1 M citric acid, 0.7 M NaCl, and 0.10 M NaCl (Control). Fitting a modified classical rate model to measurements of induction time (τ) at 20 °C for a series of supersaturated solutions, we estimate the thermodynamic barrier (ΔGc), interfacial free energy (γ), and kinetic barrier (Δagk) for silica nucleation. For 0.10 M NaCl solutions, γControl = 54.9 ± 1.6 mJ/m2 and ΔagkControl = 2.29 × 10−19 J/mol. These values are consistent with previous reports for amorphous and fused silica materials. To facilitate comparisons with the treatments, ΔagkControl is converted to a molar basis and used as a reference datum, such that ΔagkControl = 0.0 J/mol. The effects of salt and organic acids on nucleation rate have thermodynamic and kinetic origins, respectively. Faster nucleation rates measured in 0.7 M NaCl solutions arise from a lower interfacial free energy, such that γ0.7 M NaCl = 51.4 ± 1.7 mJ/m2. Organic acids increase rate through biomolecule-specific reductions in Δagk. Catalytic effects are greatest for lysine (Δagklysine = −1685 ± 315) and citric acid (Δagkcitric = −1690 ± 96 J/mol). Reductions in the kinetic barrier correlate with net positive charge of the amino acids and dissociation of the amine  $\left( {{K_{\alpha {\rm{ ‐ N}}{{\rm{H}}_3}^ {\bf{+}} }}} \right)$ group and thus the abundance of the conjugate base. Citric acid, lacking amine groups, promotes the greatest rate enhancement, thus demonstrating the role(s) of additional kinetic factors in promoting nucleation rate. Catalytic activity correlates with multiple physical and chemical properties of the organic acids.
$\left( {{K_{\alpha {\rm{ ‐ N}}{{\rm{H}}_3}^ {\bf{+}} }}} \right)$ group and thus the abundance of the conjugate base. Citric acid, lacking amine groups, promotes the greatest rate enhancement, thus demonstrating the role(s) of additional kinetic factors in promoting nucleation rate. Catalytic activity correlates with multiple physical and chemical properties of the organic acids.
To send this article to your Kindle, first ensure no-reply@cambridge.org is added to your Approved Personal Document E-mail List under your Personal Document Settings on the Manage Your Content and Devices page of your Amazon account. Then enter the ‘name’ part of your Kindle email address below. Find out more about sending to your Kindle. Find out more about saving to your Kindle.
Note you can select to save to either the @free.kindle.com or @kindle.com variations. ‘@free.kindle.com’ emails are free but can only be saved to your device when it is connected to wi-fi. ‘@kindle.com’ emails can be delivered even when you are not connected to wi-fi, but note that service fees apply.
Find out more about the Kindle Personal Document Service.
To save this article to your Dropbox account, please select one or more formats and confirm that you agree to abide by our usage policies. If this is the first time you used this feature, you will be asked to authorise Cambridge Core to connect with your Dropbox account. Find out more about saving content to Dropbox.
To save this article to your Google Drive account, please select one or more formats and confirm that you agree to abide by our usage policies. If this is the first time you used this feature, you will be asked to authorise Cambridge Core to connect with your Google Drive account. Find out more about saving content to Google Drive.