Article contents
Synthesis of Na-β″/β-Al2O3 nanorods in an ionic liquid
Published online by Cambridge University Press: 22 July 2013
Abstract
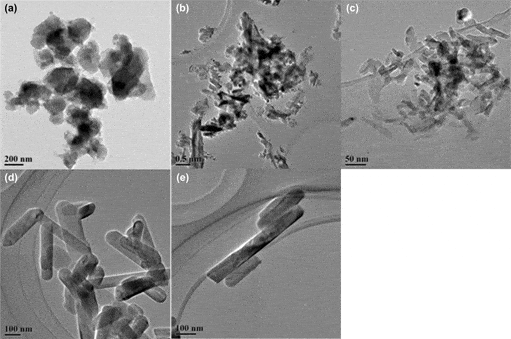
Li-stabilized Na-β″/β-Al2O3(Na1.61Li0.29Al10.70O17) nanorods were prepared by a soft chemistry process using a 1-alkyl-3-methylimidazolium bromide ([CXmim]Br, X = 4, 12, 16) ionic liquid as a template. Pure Na-β″/β-Al2O3 rods were obtained by heating at 1100 °C with [C16mim]Br as the template, resulting in nanorods of approximately 50 nm in diameter and 200–300 nm in length. It is demonstrated that alkyl chain length is the main factor determining the aspect ratio of the nanorods. The specific surface area of the powder is 81.3 m2/g, which is more than one order of magnitude higher than that of the powder prepared by a conventional solid state reaction process. The formation mechanism of the nanorods is proposed.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2013
References
REFERENCES
- 2
- Cited by


