Article contents
Synthesis of In2−xGexO3 nanopowders for thermoelectric applications
Published online by Cambridge University Press: 20 December 2011
Abstract
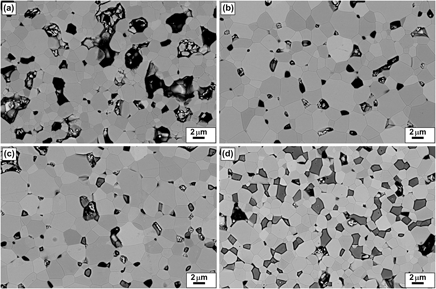
Bulk ceramics In2−xGexO3 have been synthesized in air by using citrate gel process. Nanoparticles of less than 20 nm have been synthesized through an accurate control of the processing parameters. X-ray diffraction and scanning electron microscopy studies confirmed that the solubility limit of Ge in In2O3 (xℓ) is very small and that additions of more than about 0.5 at.% Ge lead to the presence of In2Ge2O7 inclusions. Thanks to a high interdispersion of metal ions and homogeneity in elemental composition of the nanopowders obtained by citrate gel process, well-dispersed In2Ge2O7 secondary phases can be formed in the Ge-doped In2O3 matrix. An abrupt increase in the electrical conductivity and in the carrier concentration with x is observed in the monophasic region (x < xℓ), whereas in the biphasic region (x > xℓ), these values do not vary significantly. Similarly, the thermopower |S| value is correlated to this variation decreasing as x increases for x < xℓ. Above the solubility limit, the decrease in the lattice thermal conductivity is shown to be dependent on the presence of well-dispersed In2Ge207 secondary phases. The dimensionless figure of merit value is increased up to 0.3, thanks to electron doping and phonon scattering.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2011
References
REFERENCES
- 4
- Cited by


