Article contents
Synthesis and properties of SnO2 aerogels via ambient pressure drying of sol–gel
Published online by Cambridge University Press: 06 November 2018
Abstract
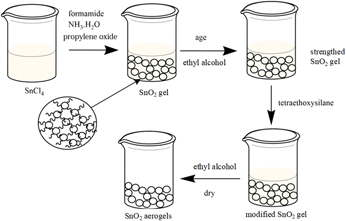
SnO2 aerogels were successfully synthesized by using SnCl4, ammonium hydroxide (AH), formamide, propylene oxide, and tetraethoxysilane as tin source, reaction promoter, drying control chemical additive (DCCA), gel accelerator, and surface modifier, respectively, via the sol–gel method, combined with subsequent ambient pressure drying of sol–gel. The results of Fourier transform infrared spectroscopy proved that the CH3CH2OSi-bond was transferred to SnO2 gel. A contact angle measuring instrument was utilized for the study of hydrophicity and hydrophobicity of SnO2 aerogels, and the complete porous skeleton structure of SnO2 aerogels was confirmed by field emission scanning electron microscopy (FESEM) and nitrogen adsorption–desorption analyzer, denoting AH allowed SnO2 aerogels to shape more macropores. Based on the thermal resistance tester, it showed that the SnO2 aerogels had low thermal conductivity. The biggest specific surface area and lowest thermal conductivity of SnO2 aerogels were 447.26 m2/g and 0.0717 K/(m•K), respectively.
- Type
- Article
- Information
- Copyright
- Copyright © Materials Research Society 2018
Footnotes
Postal address: College of Material Science and Engineering, Sichuan University, Chengdu, Sichuan, People’s Republic of China.
References
REFERENCES
- 8
- Cited by


