Article contents
Structure manufacturing of proton-conducting organic–inorganic hybrid silicophosphite membranes by solventless synthesis
Published online by Cambridge University Press: 28 February 2011
Abstract
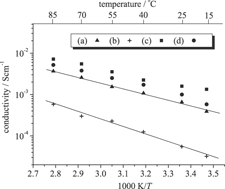
We have developed a new class of proton-conducting organic–inorganic hybrid silicophosphite membranes, produced by ethanol condensation of organically modified alkoxysilanes and anhydrous vinylphosphonic acid under solventless, catalyst-free, low-temperature, one-pot conditions. The membranes synthesized in this study are crack-free, large, and flexible, and they exhibit good thermal stability up to intermediate temperatures (~218 °C). Structural analyses using 29Si and 31P nuclear magnetic resonance spectroscopy and infrared measurements revealed that ethanol condensation produced an inorganic alternating copolymer structure, Si–O–P, with a phosphole group, and successive polymerization between vinyl and/or methacryl groups enabled these structures to connect with each other. In this way, it is possible to achieve structure manufacturing of inorganic–organic networks. The proton conductivities of the hybrids are as high as 5.2 × 10−3 S/cm at 85 °C under 80% relative humidity.
Keywords
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2011
References
REFERENCES
- 6
- Cited by


