Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Zheng, He
Wu, Shujing
Sheng, Huaping
Liu, Chun
Liu, Yu
Cao, Fan
Zhou, Zhichao
Zhao, Xingzhong
Zhao, Dongshan
and
Wang, Jianbo
2014.
Direct atomic-scale observation of layer-by-layer oxide growth during magnesium oxidation.
Applied Physics Letters,
Vol. 104,
Issue. 14,
Cao, Fan
Zheng, He
Jia, Shuangfeng
Liu, Huihui
Li, Lei
Chen, Boyun
Liu, Xi
Wu, Shujing
Sheng, Huaping
Xing, Ru
Zhao, Dongshan
and
Wang, Jianbo
2016.
Atomistic Observation of Structural Evolution during Magnesium Oxide Growth.
The Journal of Physical Chemistry C,
Vol. 120,
Issue. 47,
p.
26873.
Liu, Huihui
Zheng, He
Li, Lei
Sheng, Huaping
Jia, Shuangfeng
Cao, Fan
Liu, Xi
Chen, Boyun
Xing, Ru
Zhao, Dongshan
and
Wang, Jianbo
2017.
Atomic-scale observation of a two-stage oxidation process in Cu2O.
Nano Research,
Vol. 10,
Issue. 7,
p.
2344.
Xiang, Lijun
Guo, Jian
Wu, Chenhui
Cai, Menglei
Zhou, Xinrong
and
Zhang, Nailiang
2018.
A brief review on the growth mechanism of CuO nanowires via thermal oxidation.
Journal of Materials Research,
Vol. 33,
Issue. 16,
p.
2264.
Jia, Shuangfeng
Li, Lei
Zhao, Lulu
Zheng, He
Zhao, Peili
Guan, Xiaoxi
Chen, Guoxujia
Wu, Jiangbing
Zhou, Siyuan
and
Wang, Jianbo
2018.
Surface-dependent formation of Zn clusters in ZnO single crystals by electron irradiation.
Physical Review Materials,
Vol. 2,
Issue. 6,
Ao, Yibo
Ao, Jinqing
Yang, Xiaoshan
Zhao, Ling
Hu, Liwei
Le, Guomin
Li, Jinfeng
Qu, Fengsheng
Zhou, Yuzhao
Wang, Xiaoying
Guo, Biao
and
Liu, Xue
2020.
Preparation and characterization of hierarchical nanostructures composed by CuO nanowires within directional microporous Cu.
Vacuum,
Vol. 182,
Issue. ,
p.
109774.
Jin, Lei
Zapf, Michael
Stübinger, Martin
Kamp, Martin
Sing, Michael
Claessen, Ralph
and
Jia, Chun-Lin
2020.
Atomic‐Scale Interface Structure in Domain Matching Epitaxial BaBiO3 Thin Films Grown on SrTiO3 Substrates.
physica status solidi (RRL) – Rapid Research Letters,
Vol. 14,
Issue. 6,
Shi, Juan
Qiao, Liang
Zhao, Yi
Sun, Zhonggui
Feng, Wangjun
Zhang, Zhiya
Wang, Jun
and
Men, Xuehu
2020.
Synergistic effects on thermal growth of CuO nanowires.
Journal of Alloys and Compounds,
Vol. 815,
Issue. ,
p.
152355.
Hu, Liwei
Liu, Xue
Liang, Chuanhui
Zhao, Shaofan
Chen, Tianxiong
Li, Jinfeng
Le, Guomin
Qu, Fengsheng
Zhou, Yuzhao
Qi, Li
and
Wang, Dou
2021.
Microstructure evolution and corrosion mechanism of laser cladded Zr-Cu-Ni-Al in-situ metallic glass matrix composite coatings.
Surface and Coatings Technology,
Vol. 409,
Issue. ,
p.
126908.
Weber, Moritz L.
Wilhelm, Marek
Jin, Lei
Breuer, Uwe
Dittmann, Regina
Waser, Rainer
Guillon, Olivier
Lenser, Christian
and
Gunkel, Felix
2021.
Exsolution of Embedded Nanoparticles in Defect Engineered Perovskite Layers.
ACS Nano,
Vol. 15,
Issue. 3,
p.
4546.
Chao, Zhang
and
Dejun, Kong
2022.
Salt spray corrosion behavior and electrochemical performance of Al and Ti reinforced Ni60 coating by laser cladding.
Materials and Corrosion,
Vol. 73,
Issue. 7,
p.
1045.
Liu, Tao
Lyu, Weimin
Li, Zhicheng
Wang, Shengke
Wang, Xing
Jiang, Jiaxin
and
Jiang, Xiaosong
2023.
Recent progress on corrosion mechanisms of graphene-reinforced metal matrix composites.
Nanotechnology Reviews,
Vol. 12,
Issue. 1,
Wu, Xuelian
Wang, Yaping
Xia, Xinxin
Ding, Jianxiang
Zhang, Yundeng
Li, Gege
Ma, Chengjian
Pan, Long
Wang, Jinlong
and
Liu, Dongming
2025.
Understanding Cd Nano-Whisker Growth on Ti2CdC MAX Phase from the Perspective of Atomized Cd Migration.
JOM,
Vol. 77,
Issue. 2,
p.
822.
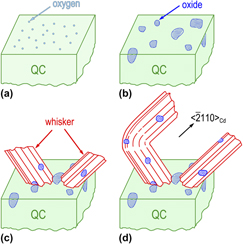
 . CdO particles are frequently observed on surfaces of whiskers and the alloy, indicating the importance of the oxidation process during the whisker growth; (ii) there exist four types of orientation relationship between Cd and CdO. The interfaces between them are shown to accommodate large lattice misfit, which is well explained by near coincidence site lattice model; (iii) nanosized Cd particles are observed to aggregate around the whisker root, providing a convincing experimental evidence for the long-range atomic diffusion. Our study offers a unique opportunity to unveil the relationship between unstability of quasicrystal structure and whisker formation and may have some implications for oxidation process of metals.
. CdO particles are frequently observed on surfaces of whiskers and the alloy, indicating the importance of the oxidation process during the whisker growth; (ii) there exist four types of orientation relationship between Cd and CdO. The interfaces between them are shown to accommodate large lattice misfit, which is well explained by near coincidence site lattice model; (iii) nanosized Cd particles are observed to aggregate around the whisker root, providing a convincing experimental evidence for the long-range atomic diffusion. Our study offers a unique opportunity to unveil the relationship between unstability of quasicrystal structure and whisker formation and may have some implications for oxidation process of metals.

