Article contents
Solvothermal synthesis and electromagnetic absorption properties of pyramidal Ni superstructures
Published online by Cambridge University Press: 15 July 2014
Abstract
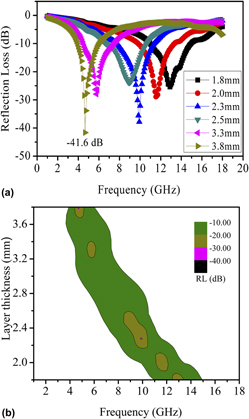
The submicrometer Ni cones have been successfully prepared through a simple solvothermal method in glycerol. The as-prepared products were extensively characterized by x-ray diffraction, field emission scanning electron microscopy, energy-dispersive x-ray spectroscopy, Fourier transform infrared spectroscopy, and thermogravimetric analysis. The effects of the volume ratios of glycerol to water, and the concentration of alkali on morphologies of Ni samples were investigated. The electromagnetic wave absorption properties of Ni cones were evaluated based on the relative complex permeability (μr) and permittivity (εr). A minimum reflection loss (RL) of −41.6 dB was observed at 4.7 GHz with the thickness of 3.8 mm and the RL values below −10 dB were obtained in the range of 3.9–15.0 GHz with the corresponding thickness of 1.8–3.8 mm. The excellent wave absorption properties of the obtained products are due to the synergic effect of dielectric loss and magnetic loss, geometry effect and unique morphology.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2014
References
REFERENCES
- 7
- Cited by


