Article contents
A simple in situ synthesis of iron oxide magnetic nanoparticles embedded in thermosensitive polymer for DNA capture
Published online by Cambridge University Press: 03 August 2020
Abstract
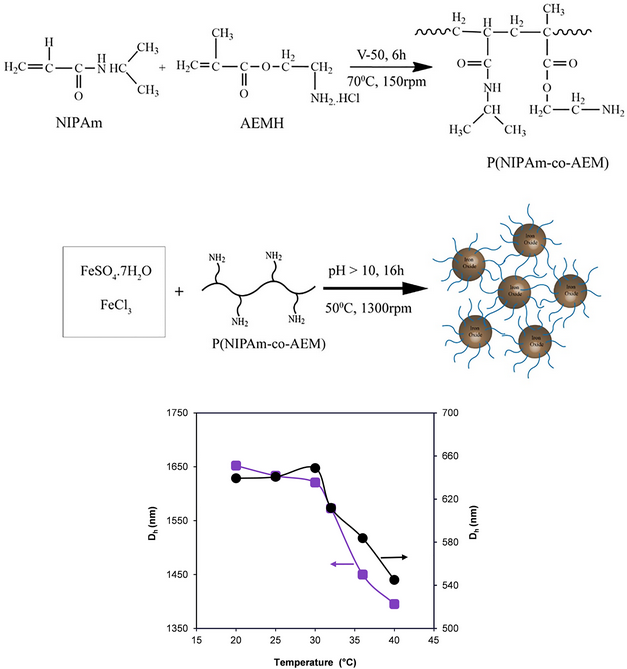
In this study, we report a simple one-pot synthesis of iron oxide nanoparticles (IONPs) modified with thermoresponsive polymers potentially applicable for nucleic acid capture. Ferrous (Fe2+) and ferric (Fe3+) ions were coprecipitated to a dispersion of previously prepared poly(N-isopropylacrylamide-co-2-aminoethyl methacrylate) P(NIPAAm-co-AEM) for in situ synthesis of magnetite (Fe3O4) and concurrent surface modification of Fe3O4 with the polymer to obtain magnetic nanocomposites. Fourier-transform infrared (FTIR) spectroscopy analysis reveals the surface modification of Fe3O4 with P(NIPAAm-co-AEM) and P(NIPAAm) as functional and control polymers, respectively. Fe3O4@P(NIPAAm-co-AEM) and Fe3O4@P(NIPAAm) nanocomposites’ surfaces contain 7.5 and 2.3 wt% of immobilized polymers, respectively. Vibrating sample magnetometry (VSM) result indicates a high saturation of magnetization value, 75 emu/g, for Fe3O4@P(NIPAAm-co-AEM) nanocomposites. The hydrodynamic diameter of Fe3O4@P(NIPAAm-co-AEM) in water changes depending on pH and temperature. A study for deoxyribonucleic acid (DNA) capture ability of Fe3O4@P(NIPAAm-co-AEM) nanocomposites shows a maximum 18.5 mg/g of DNA can be adsorbed on Fe3O4@P(NIPAAm-co-AEM).
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © Materials Research Society 2020
References
- 11
- Cited by



