Article contents
Regenerative medicine: Induced pluripotent stem cells and their benefits on accelerated bone tissue reconstruction using scaffolds
Published online by Cambridge University Press: 22 May 2018
Abstract
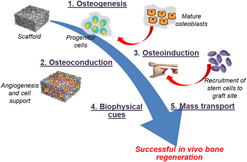
Induced pluripotent stem cells (iPSCs) offer the possibility to accelerate tissue reconstruction through cell differentiation. The use of iPSCs in bone tissue engineering is promoted by next generation scaffolds which guide bone tissue repair and provide specific cues and molecular recognition to enhance differentiation as well as the bone forming ability of these cells. However, bone tissue repair faces additional challenges such as requirement for a consequent bone vasculature and exhaustion of stem cells in the aging adults. In this context, iPSC reprogramming seems to be unaffected by age and they have better pro-angiogenic potential as well as proliferation rate. The benefits of iPSCs using polymeric scaffolds include access to humanized in vitro models, triggering bone tissue reconstruction through a supply of bone cells via differentiation, compensating mesenchymal stem cells age-related deficiencies in osteodegenerative diseases, and enhancing angiogenesis.
Keywords
- Type
- REVIEW
- Information
- Copyright
- Copyright © Materials Research Society 2018
Footnotes
This section of Journal of Materials Research is reserved for papers that are reviews of literature in a given area.
References
REFERENCES
- 7
- Cited by



