Article contents
Reactions and reversible hydrogenation of single-walled carbon nanotube anions
Published online by Cambridge University Press: 28 September 2012
Abstract
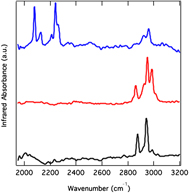
Single-walled carbon nanotube (SWNT) radical anions will react with tetrahydrofuran and generate ethylene, enolates, and a partially hydrogenated nanotube backbone. The experimental evidence suggests that there are sp3 C–H binding interactions. The total gravimetric content of hydrogen on a sample averages from 3.5% to 3.9% w/w, about four times the total amount observed for nanotubes hydrogenated via traditional Birch reduction reactions. Furthermore, the hydrogen desorbs at temperatures up to 400 °C less than those observed for the hydrogenated SWNTs formed after the Birch reduction. Finally, the first room temperature electron spin resonance spectrum of a nanotube radical ion is also reported.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2012
References
REFERENCES
- 1
- Cited by


