Published online by Cambridge University Press: 19 August 2013
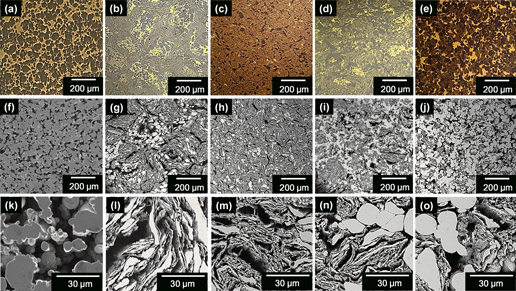
Among the different porous materials, bulk metallic glass (BMG) foams are of special interest due to their high strength combined with large elastic limit. Large surface areas and, therefore, high reactivity in chemical applications can be achieved by properly adjusting the pore characteristics. Pore size and pore size distribution are the key factors for determining the overall performance of open-cell porous materials used for functional applications, such as filtration or catalysis. As a result, the control of these factors is a necessary requirement for material design and application. In this work, BMG foams are produced by powder metallurgy through the selective dissolution of a fugitive phase. The work is focused on the manufacturing processes needed to properly control pore size and pore size distribution. The results reveal that customized hybrid BMG porous structures can be produced through the controlled milling of the BMG-composite powders.