Article contents
Preparation of TiO2-(B)/SnO2 nanostructured composites and its performance as anodes for lithium-ion batteries
Published online by Cambridge University Press: 14 September 2020
Abstract
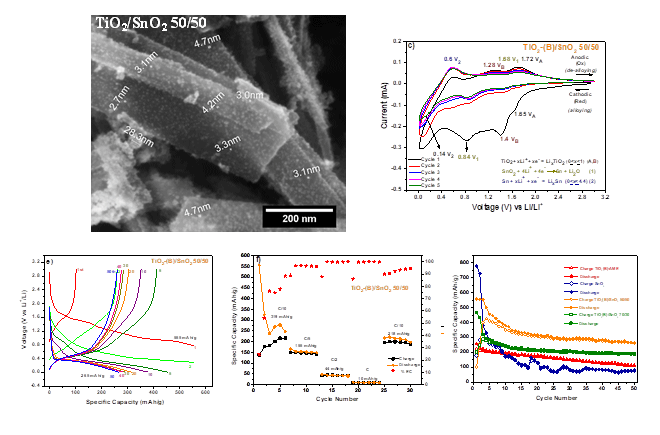
TiO2-(B)/SnO2 nanostructured composites have been prepared by the combination of an oil-in-water (O/W) microemulsion reaction method (MRM) and a hydrothermal method. Its electrochemical properties were investigated as anode materials in lithium-ion battery, and characterization was carried out by XRD, BET, Raman, FE-SEM, EDXS, and TEM. The as-prepared composites consisted of monoclinic phase TiO2-(B) nanoribbons decorated with cassiterite structure SnO2 nanoparticles. The electrochemical performance of the TiO2-(B)/SnO2 50/50 nanocomposite electrode showed higher reversible capacity of 265 mAh/g than that of the pure SnO2 electrode, 79 mAh/g, after 50 cycles at 0.1 C in a voltage range of 0.01-3.0 V at room temperature. In addition, the coulombic efficiency of the TiO2-(B)/SnO2 50/50 nanocomposite remains at an average greater than 90% from the 2nd to the 50th cycles. The TiO2-(B)/SnO2 50/50 nanocomposite presented the best balance between the mechanical support effect provided by TiO2-(B) that also contributes to the LIB capacity and the SnO2 that provides high specific capacity.
- Type
- Invited Feature Paper
- Information
- Copyright
- Copyright © Materials Research Society 2020
References
- 9
- Cited by



