Article contents
Preparation of core–shell nanostructured black nano-TiO2 by sol–gel method combined with Mg reduction
Published online by Cambridge University Press: 16 November 2018
Abstract
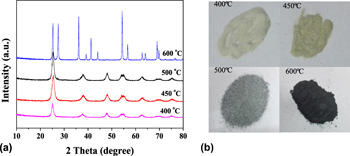
Black nano-TiO2 samples with core–shell nanostructure were successfully prepared by sol–gel method combined with Mg reduction using butyl titanate as titanium source and calcining at 500°C in air atmosphere and at 400–600°C in nitrogen atmosphere. The prepared black TiO2 samples were characterized by X-ray diffraction, high resolution transmission electron microscopy, Raman spectra, photoluminescence emission spectra, N2 adsorption–desorption, and ultraviolet–visible spectroscopy. The results show that the black TiO2 exhibits a crystalline core–disordered shell structure composed of disordered surface and oxygen vacancies, and the thickness of the disordered layer is about 2–3 nm. The optical absorption properties of black nano-TiO2 samples have been remarkably enhanced in visible light region. Compared with the white TiO2, the reduced black TiO2 samples exhibit enhanced photocatalytic hydrogen production under the full solar wavelength range of light, and the sample prepared with the Mg and TiO2 ratio of 9:1 calcined at 500 °C has the maximum hydrogen production rate.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © Materials Research Society 2018
References
REFERENCES
- 8
- Cited by


