Article contents
Ni–Co composite metal embedded porous N-doped carbon for an effective binder-free supercapacitor electrode
Published online by Cambridge University Press: 18 December 2017
Abstract
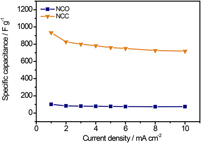
Binary transition metal oxides, such as NiCo2O4 (NCO) electrode have been demonstrated to be promising candidates for high-performance supercapacitors. However, their low electrical conductivity and poor stability made the electrochemical performance of most current NCO electrodes yet below the expectation. Herein, a novel electrode (NCC) consisting of binary transition metal (Ni–Co) nanoparticles embedded N-doped porous carbon matrix on graphite papers (GPs) has been developed with a high specific capacitance of 933.5 F/g at 1 mA/cm2 which is substantially 10 times than that of the NCO electrode (99.3 F/g) and much higher than those of most reported NCO based electrode. Moreover, this NCC electrode has an ultrahigh rate capability of 725.5 F/g at 10 mA/cm2 with excellent electrochemical durability (no capacitance decreases after 10,000 cycles). These results indicate a promising potential application of Ni–Co metal composite embedded carbon matrix for using as an effective electrode material in supercapacitors.
Keywords
- Type
- Article
- Information
- Journal of Materials Research , Volume 33 , Issue 9: Focus Issue: Porous Carbon and Carbonaceous Materials for Energy Conversion and Storage , 14 May 2018 , pp. 1167 - 1178
- Copyright
- Copyright © Materials Research Society 2017
Footnotes
Contributing Editor: Tianyu Liu
References
REFERENCES
- 8
- Cited by



