Article contents
Monodisperse Au/aminosilica composite nanospheres: Facile one-step synthesis and their applications in gene transfection
Published online by Cambridge University Press: 11 July 2012
Abstract
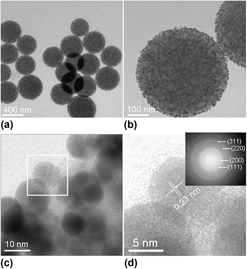
In this study, Au/aminosilica composite nanospheres have been synthesized via a simple one-pot route using HAuCl4 and N-(3-trimethoxysilylpropyl)-ethylenediamine as starting materials. Scanning electron microscopy results show that these spheres are with diameters of about 300 nm. The obtained Au/aminosilica nanospheres were used as nonviral carriers for gene delivery. Compared with commercial Lipofectamine 2000, the Au/aminosilica nanospheres are with higher transfection efficiency and lower cytotoxicity. Furthermore, the nanospheres are biocompatible, which may find applications in gene delivery and drug carrier.
Keywords
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2012
References
REFERENCES
- 4
- Cited by


