Article contents
Mono and dialkoxysilane surface modification of superparamagnetic iron oxide nanoparticles for application as magnetic resonance imaging contrast agents
Published online by Cambridge University Press: 03 July 2012
Abstract
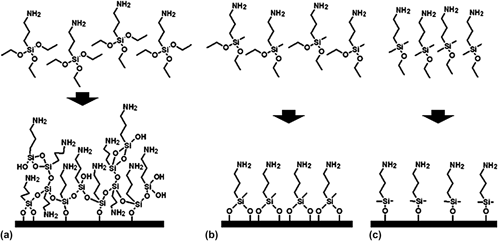
In this study, we have developed and characterized two previously unstudied alkoxysilane surface chemistries for use with superparamagnetic iron oxide (SPIO) nanoparticles as a magnetic resonance imaging contrast agent. We modified superparamagnetic iron oxide nanoparticles (SPIO) using aminopropyl triethoxysilane and two analogous alkoxysilanes, aminopropyl dimethylethoxysilane and aminopropyl methyldiethoxysilane, to compare a mono- and dialkoxysilane, respectively, to a more commonly used trialkoxysilane as two new SPIO surface chemistries capable of forming ultrathin functional surface coatings. The ligand densities of the mono- and dialkoxysilane-modified SPIO produced in this study are consistent with near monolayers of ligands on the SPIO surface. We studied the chemical stability of the mono-, di-, and trialkoxysilane-modified SPIO in neutral and acidic media to evaluate the viability of these surface chemistries for use in long-term intracellular applications. The mono- and dialkoxysilane-modified SPIO demonstrate comparable chemical stability to the trialkoxysilane-modified SPIO, indicating that the mono- and dialkoxysilane are both viable new SPIO surface chemistries for future applications requiring minimally thick alkoxysilane surface coatings.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2012
References
REFERENCES
- 8
- Cited by


