Article contents
Mechanism and kinetics of stabilization reactions of poly(acrylonitrile-co-β-methylhydrogen itaconate)
Published online by Cambridge University Press: 16 July 2012
Abstract
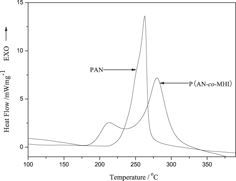
To replace ternary polymer with binary polymer, a novel bifunctional comonomer β-methylhydrogen itaconate (MHI) was synthesized to prepare poly(acrylonitrile-co-β-methylhydrogen itaconate) [P(AN-co-MHI)] copolymer used for carbon fiber precursor. The structural evolution and stabilization mechanism of P(AN-co-MHI) and polyacrylonitrile (PAN) during stabilization were studied by Fourier transform infrared spectroscopy, x-ray diffraction, differential scanning calorimetry, thermogravimetry, and kinetic investigation. The activation energy (Ea) of the stabilization reactions were calculated by Kissinger method and Ozawa method. The results show that the P(AN-co-MHI) exhibits a lower stabilization temperature than terpolymers containing similar chemistry component and a significantly improved stabilization performance compared with PAN homopolymer, such as lower cyclization temperature, lower Ea (85.36 kJ/mol), and larger extent of stabilization under the same condition, which is mainly attributed to the initiation of MHI comonomer through an ionic cyclization mechanism. The dehydrogenation of P(AN-co-MHI) is also promoted by the incorporation of comonomer MHI into PAN chains. Simultaneously, the rheological analysis shows that P(AN-co-MHI) possesses better spinnability than PAN, which is beneficial for preparation of high performance carbon fiber.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2012
References
REFERENCES
- 9
- Cited by


