Article contents
Kinetic control of CeO2 nanoparticles for catalytic CO oxidation
Published online by Cambridge University Press: 11 February 2019
Abstract
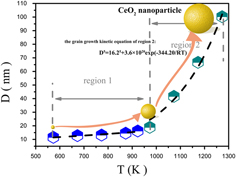
This article reports on the growth kinetics of cerium oxide (CeO2) nanoparticles prepared via a sintering method. By varying the sintering temperatures and periods of time, particle size of CeO2 nanoparticles was tuned from 11 to 100 nm. Ostwald ripening mechanism prevails in the growth process, and the growth kinetics is determined to follow an equation, D5 = 16.25 + 3.6 × 1020 exp(−344.20/RT) in the temperature range of 700 to 1000°C. After dispersing Pt on CeO2 nanoparticles, the size effect for the catalytic performance of the CO oxidation reaction was researched. When temperature and period of time are set at 700 °C and 2 h, respectively, dispersion of Pt onto CeO2 nanoparticles led to the largest quantity of chemisorbed oxygen species on the surface and the best catalytic performance. The findings reported here would provide a feasible path for the preparation of advanced catalysts in the future and moreover to discover novel size-dependent supports for many catalytic applications.
Keywords
- Type
- Invited Feature Paper
- Information
- Journal of Materials Research , Volume 34 , Issue 13: Focus Issue: Intrinsic and Extrinsic Size Effects in Materials , 15 July 2019 , pp. 2201 - 2208
- Copyright
- Copyright © Materials Research Society 2019
Footnotes
This paper has been selected as an Invited Feature Paper.
References
- 11
- Cited by


