Article contents
Investigation on the properties of hybrid CH3NH3SnxI3 (0.9 ≤ x ≤ 1.4) perovskite systems
Published online by Cambridge University Press: 07 November 2017
Abstract
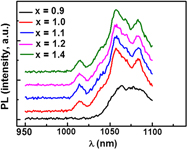
Methylammonium-tin-iodide (MASnxI3, 0.9 ≤ x ≤ 1.4) systems were prepared by self assembly process in aqueous solutions. The “as-prepared” MASnxI3 systems exhibit a crystalline tetragonal structure (space group I4cm) with polyhedral-shaped crystallites. The as-prepared samples were annealed at T = 150 °C, t = 8 h under nitrogen and synthetic air. Under nitrogen, the CH3NH3SnxI3 systems adopted a cubic crystalline structure (space group P4mm) with crystallites of 2–4 μm length, whereas under air, the formation of noncrystalline phases was observed. The optical absorption spectra displayed absorption edges at 1107.0 nm (x = 0.9), 1098.6 nm (x = 1.0), and 1073.2 nm (x = 1.1), respectively, whereas at higher Sn-content (x ≥ 1.2), a broad tail of the absorbance profile was observed. The photoluminescence (PL) emission spectra (RT, λexc = 500 nm) showed major PL-events over 1 µm range and the appearance of additional bands at increasing the Sn-content. The fabrication of layers with a semiconducting behavior was demonstrated.
Keywords
- Type
- Invited Paper
- Information
- Copyright
- Copyright © Materials Research Society 2017
Footnotes
Contributing Editor: Sam Zhang
References
REFERENCES
- 6
- Cited by



