Article contents
Investigation of grafted mesoporous silicon sponge using hyperpolarized 129Xe NMR spectroscopy
Published online by Cambridge University Press: 19 July 2018
Abstract
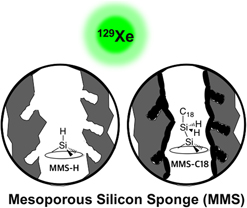
Temperature-dependent (173–373 K) hyperpolarized 129Xe nuclear magnetic resonance (129Xe NMR) analyses along with transmission electron microscopy and N2 adsorption measurements have been applied to understand pore structure and interconnectivity of bare and grafted mesoporous silicon sponge (MSS) materials. The Xe NMR chemical shift data indicate the existence of micropores inside the larger mesopore channels and the effects of grafting on the pore surfaces. The grafted layer estimated at 2 nm in thickness blocks the micropores on the surfaces of mesoporous channels. Partitioning of Xe between the micropores and the mesopores in the MSS materials is temperature-dependent, with Xe principally occupying the micropores at lower temperatures. In addition, the temperature-dependent Xe peak shift of MSS materials verifies the increased uniformity and interconnectivity of mesopores after surface grafting. The results from this study provide useful information for design and development of novel materials.
- Type
- Article
- Information
- Copyright
- Copyright © Materials Research Society 2018
References
REFERENCES
- 4
- Cited by


