Published online by Cambridge University Press: 18 September 2017
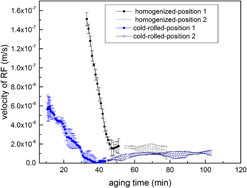
Age-hardening of homogenized and cold-rolled invar-based Sn alloys results in the development of continuously-formed (CP) and discontinuously-formed (DP) Ni3Sn2 precipitates. In situ investigation of the DP reaction front (RF) velocity (V) revealed a nonsteady-state behavior upon early aging stages followed by a constant V after prolonged aging. The reason for the initial nonsteady-state behavior was experimentally studied and attributed to the reduction of matrix Sn-supersaturation ahead of the DP RF as a result of the simultaneous CP coarsening (in homogenized specimen) or the CP increased volume fraction (in cold-rolled specimen). A similar trend of V variation in the homogenized specimen was obtained after the modification of the original Hornbogen model for the nonsteady-state DP growth kinetics. In general, variations of the transformed matrix fraction via the DP reaction suggest the faster kinetics of this reaction in cold-rolled specimen as compared to the homogenized one due to the existence of more nucleation sites induced by the cold deformation.
Contributing Editor: Jürgen Eckert