Article contents
Influence of the anion nature on the properties of Sr-containing formamidinium tin halide perovskites
Published online by Cambridge University Press: 09 November 2018
Abstract
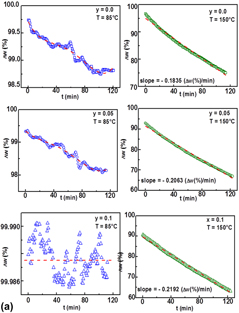
Formamidinium–tin–strontium halides (CH(NH2)2Sn1−ySryX3 (FASnX3), X = I, Br and 0.0 ≤ y ≤ 0.1) were investigated. X-ray diffraction analysis revealed orthorhombic FASnI3 (space group Amm2) and SnI2 for X = I as well as cubic FASnBr3 (space group  $Pm\bar{3}m$) and SnBr2 for X = Br, respectively. For X = I, the optical spectra displayed a decrease of the absorption edges with increasing Sr content (1055 nm, y = 0.0; 950–960 nm, y > 0.0) and a direct semiconducting behavior with narrow band energy gaps (1.31–1.34 eV). For X = Br, on increasing the absorption edges (492 nm, y = 0.0; 975 nm, y = 0.075), a direct semiconducting behavior with band energy gaps between 2.65 eV (y = 0.0) and 1.38 eV (y = 0.075) were observed and the emission photoluminescence (PL) spectra (excitation wavelength λexc = 380 nm) showed an increase of the luminescence response after the thermal treatment.
$Pm\bar{3}m$) and SnBr2 for X = Br, respectively. For X = I, the optical spectra displayed a decrease of the absorption edges with increasing Sr content (1055 nm, y = 0.0; 950–960 nm, y > 0.0) and a direct semiconducting behavior with narrow band energy gaps (1.31–1.34 eV). For X = Br, on increasing the absorption edges (492 nm, y = 0.0; 975 nm, y = 0.075), a direct semiconducting behavior with band energy gaps between 2.65 eV (y = 0.0) and 1.38 eV (y = 0.075) were observed and the emission photoluminescence (PL) spectra (excitation wavelength λexc = 380 nm) showed an increase of the luminescence response after the thermal treatment.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © Materials Research Society 2018
References
REFERENCES
- 6
- Cited by


