Article contents
Influence of heat treatment on the microstructure and corrosion behavior of Ni–Fe–Cr alloy 028
Published online by Cambridge University Press: 26 September 2014
Abstract
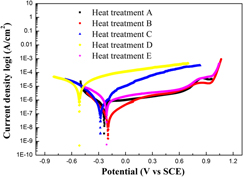
To thoroughly understand the relationship between heat treatments and characteristics of both microstructure and corrosion behavior of Ni–Fe–Cr alloy 028, a series of heat treatments were carried out. The area fraction of precipitates increases with increasing duration of aging treatment at 900 °C. The precipitation rate is higher at 900 °C than at 850 and 950 °C. The precipitates are formed both in grains and at grain boundaries, this behavior enhances the hardness. The corrosion behavior was evaluated by potentiodynamic polarization test and electrochemical impedance spectroscopy measurement under sodium chloride solution. The results indicate that the variation of morphology, amount, and distribution of precipitates attributed to the heat treatment strongly influences the corrosion behavior of alloy 028 in the sodium chloride solution. There is a galvanic effect of Cr-rich phase in the corrosion process. The increase of corrosion rate with the aging time is attributed to the acceleration of the microgalvanic corrosion.
Keywords
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2014
References
REFERENCES
- 5
- Cited by


