Article contents
In situ hydrothermal synthesis of rGO-wrapped Fe1−xS particles for lithium storage
Published online by Cambridge University Press: 23 September 2019
Abstract
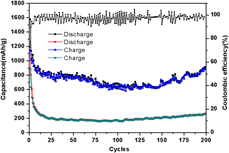
Iron sulfides have attracted much interests for their potential as anode materials in energy storage devices in view of their low costs, and environmentally benign and high theoretical capacities. Among them, Fe1−xS is relatively rarely investigated. In this work, Fe1−xS@rGO has been synthesized using a facile in situ hydrothermal method. After wrapped by rGO, the morphology of Fe1−xS particles changes from hexagonal flakes to irregular particles with much smaller sizes. As the anode material for lithium ion batteries, Fe1−xS@rGO exhibits excellent lithium storage ability. It can deliver an initial discharge capacity of 1575.5 mA h/g in the potential window of 0.005–3 V, and a reversible capacity of 907.8 mA h/g can be maintained after 200 cycles at 100 mA/g. Its improved electrochemical performance can be attributed to the effect of enhanced contact area and shortened Li+ ion transport distance because of rGO’s contribution.
- Type
- Article
- Information
- Copyright
- Copyright © Materials Research Society 2019
References
- 8
- Cited by


