Published online by Cambridge University Press: 21 September 2020
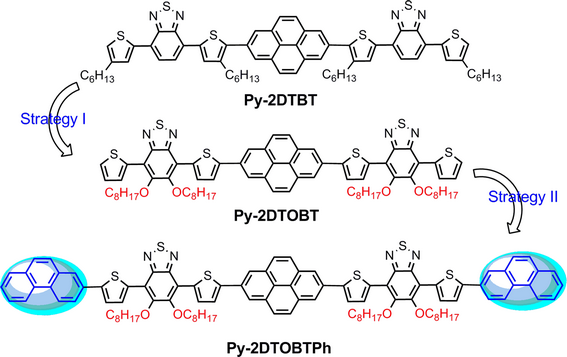
A–Ar–A-type small molecule (SM) of Py-2DTOBT and Py-2DTOBTPh with an Ar(A–D)2 framework were synthesized, in which 2,7-pyrene (Py) and alkoxyl-substituted benzothiadiazole (OBT) were, respectively, used as the central aryl (Ar) and arm acceptor (A), while 3-phenanthrene (Ph) was used as a terminal donor (D) in Py-2DTOBTPh. By comparison with the parent SM of Py-2DTBT, where 2,7-pyrene (Py) and benzothiadiazole (BT) were used as the central aryl (Ar) and arm acceptor (A), the effects of non-covalent interactions and the terminal group on optical, electrochemical, and photovoltaic properties were investigated. The gradually improved photovoltaic performances were observed among Py-2DTBT, Py-2DTOBT, and Py-2DTOBTPh based organic solar cells. A power conversion efficiency (PCE) of 2.83% was obtained in the Py-2DTOBTPh/PC71BM-based device, which is a 53% improvement related to that of Py-2DTOBT and three times enhanced related to that of Py-2DTBT(Py-2DTOBT:PCE of 1.86%, Py-2DTBT:PCE of 0.74%).