Article contents
Hydrothermal zinc oxide nanowire growth with different zinc salts
Published online by Cambridge University Press: 02 August 2012
Abstract
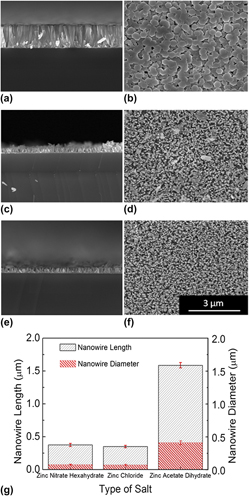
The effect of the use of different zinc salts as zinc sources during hydrothermal growth of zinc oxide nanowires was systematically investigated. Change in the temperature, pH, and transmittance of the growth solutions prepared with three different zinc salts was monitored and used to provide a broad explanation to the effect of the salt. In addition to conventional heating process, microwave heating of the growth solutions was also performed, and differences in the ZnO nanowires synthesized through both heating methods were examined. It was found that ionization of zinc in growth solutions is influencing the formation of ZnO nanowires leading to growth with different aspect ratios, and zinc acetate dihydrate salt allows the synthesis of nanowires with the highest aspect ratio.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2012
References
REFERENCES
- 7
- Cited by


