Published online by Cambridge University Press: 17 January 2017
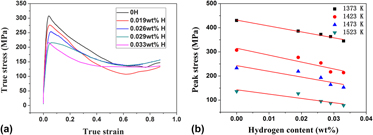
The hydrogenation behavior of Ti–44Al–6Nb (at.%) alloy was studied at temperature range of 1373–1693 K, and the effect of hydrogen on hot deformability was tested on Gleeble-1500D thermo-simulation machine. It is found that the lnCH increases linearly with 1/T, and hydrogen content increases with increasing of hydrogen time and flow rate logarithmically. The positive heat of solution of hydrogen denotes that hydrogen absorption in TiAl alloys is an endothermic reaction. The results also show that hydrogen promotes the lamellar colony size and lamellar spacing because that hydrogen can promote the diffusion of elements. There is more residual B2 phase in the hydrogenated alloy revealing that hydrogen stabilizes the B2 phase during hydrogenation. The nanohardness and elastic modulus of the alloy are decreased from 4.4 and 213.5 GPa to 4.2 and 199.8 GPa after hydrogenation with 0.033 wt% H. Thermal simulation results show that the peak stress is decreased by 30% after hydrogenation with 0.033 wt% H which corresponds to decreasing the deformation temperature by about 50 K. This is attributed to hydrogen-promoted dynamic recrystallization and dislocation movement.
Contributing Editor: Jürgen Eckert