Article contents
A feasible route to prepare hollow ZnO microtube via modulating reagent’s vapor pressure and growth temperature
Published online by Cambridge University Press: 23 January 2013
Abstract
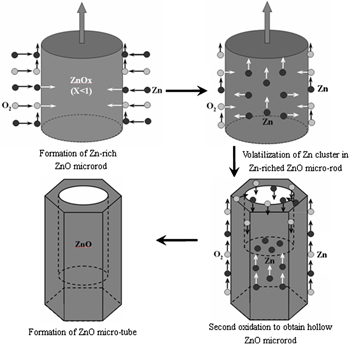
In this article, ZnO microtube was prepared using mixed powder of Zn, ZnO, and carbon as source via chemical vapor deposition method. The growth process was discussed in detail, and the high Zn vapor pressure and high growth temperature were considered as two crucial factors determining the formation of tubular structure. A two-step growth model was proposed, namely initial deficient-oxidation and followed by second-volatilization. Four another experiments were further conducted to analyze the growth behavior of reagent species under different Zn vapor pressure and growth temperature, respectively. These experimental results indicated that the formation of Zn-rich structure under enough high Zn vapor pressure and second-volatilization of these abundant interstitial Zn under high growth temperature were important to form tubular structure. Our experimental method provided a feasible route to prepare other hollow structures, such as oxide, sulfide, etc. Furthermore, these synthesized ZnO microtube might have potential application as functional blocks in nanodevices.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2013
References
REFERENCES
- 1
- Cited by


