Article contents
Facile synthesis of hydrodynamic solid lubricant MoS2 from molybdenum trioxide nanorods
Published online by Cambridge University Press: 03 July 2013
Abstract
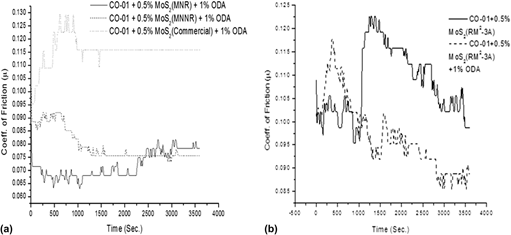
Molybdenum trioxide nanostructures were synthesized, to make highly friction resistant molybdenum disulfide, by low temperature hydrothermal reaction without using any template or catalyst. The as-synthesized materials were sulfided with H2S at 400 and 800 °C. X-ray diffraction, scanning electron microscopy, transmission electron microscopy, Fourier-transform infra-red spectroscopy, and thermogravimetric analysis were used for the physical characterization of the materials. The oxide material is highly crystalline with unique morphology. The factors affecting the size and shape of the synthesized materials were studied in detail. The crystalline nature of the materials decreased after the sulfidation process at 800 °C without any change in morphology. The wear resistance and lubricity of the material were studied under harsh conditions. The comparative study of these materials with MoS2 prepared by the hard templating method (using mesoporous silica template) reveals that the new material synthesized by direct hydrothermal route is pure phase and has better wear resistance and antifriction properties. Ultra high stability of the material is the most distinguished property of the material synthesized.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2013
Footnotes
These authors contributed equally to this work.
References
REFERENCES
- 3
- Cited by


