Article contents
Fabrication and determination of growth regimes of various Pd NPs based on the control of deposition amount and temperature on c-plane GaN
Published online by Cambridge University Press: 17 July 2017
Abstract
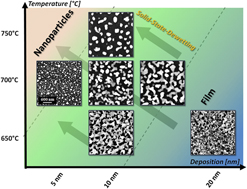
Palladium (Pd) nanostructures have been actively adapted for various applications and their properties and applicability closely depend on their shape, size, and density. In this paper, the evolution of self-assembled Pd nanostructures on the hexagonal c-plane GaN is presented by the systematical control of Pd deposition amount (DA) at distinctive temperatures. Pd nanostructures of various configurations, sizes, and densities are demonstrated based on the solid-state dewetting of Pd thin films and a clear distinction in the growth regimes is observed. Three growth regimes are clearly observed depending on the variation of DA, i.e., (i) the agglomeration of Pd nanoparticles, (ii) the coalescence of wiggly Pd nanostructures, and finally (iii) the growth of nanovoids and layers. Owing to the temperature-dependent dewetting process, the growth regimes are markedly shifted, resulting in the distinctive Pd nanostructures within the identical DA range. The results are discussed in conjunction with the surface diffusion, Volmer–Weber and coalescence growth model, and surface/interface energy minimization mechanism. In addition, the evolution of optical properties, emission band, and lattice properties are probed by reflectance, photoluminescence, and Raman spectroscopy, which exhibit varying spectral intensity and peak positions according to the surface morphology of Pd nanostructures.
Keywords
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2017
Footnotes
Contributing Editor: Jürgen Eckert
References
REFERENCES
- 2
- Cited by



