Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Sereda, V. V.
Sednev-Lugovets, A. L.
Malyshkin, D. A.
Ivanov, I. L.
Tsvetkov, D. S.
and
Zuev, A. Yu.
2020.
Thermodynamics of BaCa(1 + y)/3Nb(2 − y)/3O3 − δ·xH2O proton-conducting perovskites.
Journal of Thermal Analysis and Calorimetry,
Vol. 142,
Issue. 5,
p.
1989.
Tamai, Kazuki
Hosokawa, Saburo
Kato, Kazuo
Asakura, Hiroyuki
Teramura, Kentaro
and
Tanaka, Tsunehiro
2020.
Low-temperature NO oxidation using lattice oxygen in Fe-site substituted SrFeO3−δ.
Physical Chemistry Chemical Physics,
Vol. 22,
Issue. 42,
p.
24181.
Bulfin, B.
Buttsworth, L.
Lidor, A.
and
Steinfeld, A.
2021.
High-purity nitrogen production from air by pressure swing adsorption combined with SrFeO3 redox chemical looping.
Chemical Engineering Journal,
Vol. 421,
Issue. ,
p.
127734.
Ni, Chengsheng
Zhou, Jun
Zhang, Ziye
Li, Shuangbin
Ni, Jiupai
Wu, Kai
and
Irvine, John T. S.
2021.
Iron-based electrode materials for solid oxide fuel cells and electrolysers.
Energy & Environmental Science,
Vol. 14,
Issue. 12,
p.
6287.
Sereda, Vladimir V.
Malyshkin, Dmitry A.
Ivanov, Ivan L.
Tsvetkov, Dmitry S.
Zuev, Andrey Yu.
and
Maignan, Antoine
2021.
Redox Thermochemistry, Thermodynamics, and Solar Energy Conversion and Storage Capability of Some Double Perovskite Cobaltites.
Inorganic Chemistry,
Vol. 60,
Issue. 23,
p.
18141.
Malyshkin, Dmitry A.
Sereda, Vladimir V.
Sednev-Lugovets, Anton L.
Ivanov, Ivan L.
Tsvetkov, Dmitry S.
and
Zuev, Andrey Yu.
2022.
Defect-Induced Properties and Thermodynamics of La0.5Ba0.5CoO3–δ
.
Journal of The Electrochemical Society,
Vol. 169,
Issue. 2,
p.
024511.
Nguyen, Hien Thi Dieu
Wang, Yu
Schoenherr, Peggy
Sharma, Pankaj
and
Seidel, Jan
2022.
“Oxygen Sponge” Dynamics in Topotactic SrCo1–xFexO3−δ.
ACS Applied Electronic Materials,
Vol. 4,
Issue. 12,
p.
6382.
Patankar, Aniket S.
Wu, Xiao-Yu
Choi, Wonjae
Tuller, Harry L.
and
Ghoniem, Ahmed F.
2023.
A comparative analysis of integrating thermochemical oxygen pumping in water-splitting redox cycles for hydrogen production.
Solar Energy,
Vol. 264,
Issue. ,
p.
111960.
Liu, Junchen
and
Li, Fanxing
2023.
Mixed oxides as multi-functional reaction media for chemical looping catalysis.
Chemical Communications,
Vol. 59,
Issue. 1,
p.
10.
Capstick, S.
Bulfin, B.
Naik, J.M.
Gigantino, M.
and
Steinfeld, A.
2023.
Oxygen separation via chemical looping of the perovskite oxide Sr0.8Ca0.2FeO3 in packed bed reactors for the production of nitrogen from air.
Chemical Engineering Journal,
Vol. 452,
Issue. ,
p.
139289.
Wilson, Steven A.
Stechel, Ellen B.
and
Muhich, Christopher L.
2023.
Overcoming significant challenges in extracting off-stoichiometric thermodynamics using the compound energy formalism through complementary use of experimental and first principles data: A case study of Ba1-xSrxFeO3-δ.
Solid State Ionics,
Vol. 390,
Issue. ,
p.
116115.
Ivanov, Aleksei I.
Nikitin, Sergey S.
Dyakina, Mariya S.
Tsipis, Ekaterina V.
Patrakeev, Mikhail V.
Agarkov, Dmitrii A.
Zverkova, Irina I.
Zhigachev, Andrey O.
Kedrov, Victor V.
and
Kharton, Vladislav V.
2025.
Oxygen Nonstoichiometry, Electrical Conductivity, Chemical Expansion and Electrode Properties of Perovskite-Type SrFe0.9V0.1O3−δ.
Materials,
Vol. 18,
Issue. 3,
p.
493.
Sereda, Vladimir V.
Tsvetkov, Dmitry S.
Sereda, Anna V.
Malyshkin, Dmitry A.
Ivanov, Ivan L.
and
Zuev, Andrey Yu.
2025.
Enthalpy increments and redox energetics of titanium-substituted strontium ferrites SrTi1-xFexO3-δ.
Journal of Alloys and Compounds,
Vol. 1010,
Issue. ,
p.
177780.
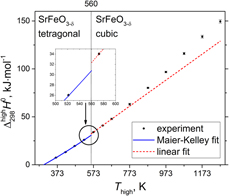
 $\Delta _{298}^T{H^0}$, for highly nonstoichiometric SrFeO3−δ (δ = 0.18–0.41) were obtained between 373 and 1273 K in air using drop calorimetry. The analysis of the
$\Delta _{298}^T{H^0}$, for highly nonstoichiometric SrFeO3−δ (δ = 0.18–0.41) were obtained between 373 and 1273 K in air using drop calorimetry. The analysis of the  $\Delta _{298}^T{H^0}\left( T \right)$ dependence at lower temperatures allowed evaluating the enthalpy of tetragonal to cubic
$\Delta _{298}^T{H^0}\left( T \right)$ dependence at lower temperatures allowed evaluating the enthalpy of tetragonal to cubic  ${{I4} / {mmm}} \,{\tf="TeX CM Bold Maths Symbols"\char33}\, Pm\bar{3}m$ phase transition at 560 K, 1.57 kJ/mol, and the Maier–Kelley function for
${{I4} / {mmm}} \,{\tf="TeX CM Bold Maths Symbols"\char33}\, Pm\bar{3}m$ phase transition at 560 K, 1.57 kJ/mol, and the Maier–Kelley function for  $\Delta _{298}^T{H^0}\left( T \right)$ of tetragonal SrFeO3−δ (space group
$\Delta _{298}^T{H^0}\left( T \right)$ of tetragonal SrFeO3−δ (space group  ${{I4} / {mmm}}$). Combined investigation of oxygen nonstoichiometry
${{I4} / {mmm}}$). Combined investigation of oxygen nonstoichiometry  $\bolddelta \left( T \right)$ dependence, measured by thermogravimetry, and higher-temperature
$\bolddelta \left( T \right)$ dependence, measured by thermogravimetry, and higher-temperature  $\Delta _{298}^T{H^0}\left( T \right)$ of cubic SrFeO3−δ (space group
$\Delta _{298}^T{H^0}\left( T \right)$ of cubic SrFeO3−δ (space group  $Pm\bar{3}m$) yielded the temperature-dependent reduction (oxygen release) enthalpy,
$Pm\bar{3}m$) yielded the temperature-dependent reduction (oxygen release) enthalpy,  $\Delta H_{{\rm{red}}}^{\rm{0}}$. Calorimetrically-determined
$\Delta H_{{\rm{red}}}^{\rm{0}}$. Calorimetrically-determined  $\Delta H_{{\rm{red}}}^{\rm{0}}$ of SrFeO3−δ increases from 65 ± 7 kJ/mol O at 873–973 K to 84 ± 7 kJ/mol O at 1073–1273 K, which may indicate that the short-range vacancy ordering in SrFeO3−δ is hampered at higher temperatures.
$\Delta H_{{\rm{red}}}^{\rm{0}}$ of SrFeO3−δ increases from 65 ± 7 kJ/mol O at 873–973 K to 84 ± 7 kJ/mol O at 1073–1273 K, which may indicate that the short-range vacancy ordering in SrFeO3−δ is hampered at higher temperatures.