Article contents
Enthalpies of formation of the solid solutions of ZrxY0.5−x/2Ta0.5−x/2O2 (0 ≤ x ≤ 0.2 and 0.65 ≤ x ≤ 1)
Published online by Cambridge University Press: 29 July 2019
Abstract
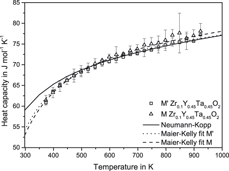
Enthalpies of formation and heat capacities are key parameters for thermodynamic assessments using the CALPHAD method and were determined in this work in the ZrO2–Y0.5Ta0.5O2 quasibinary system. ZrxY0.5−x/2Ta0.5−x/2O2 samples with compositions in the monoclinic and tetragonal ZrO2-based solid solutions (0.65 ≤ x ≤ 1) as well as the two monoclinic (M′ and M) polymorphs of the Y0.5Ta0.5O2-based solid solutions (0 ≤ x ≤ 0.2) were investigated. The enthalpies of the two monoclinic polymorphs M′ and M are essentially the same within experimental uncertainty and have significant energetic stability (enthalpies of formation near −45 kJ/mol) relative to their component oxides. The monoclinic and tetragonal zirconia-rich solid solutions have little energetic stability with respect to ZrO2, Ta2O5, and Y2O3 and presumably owe their existence to the configurational entropy. The heat capacities of M′ and M phase are similar and can be estimated from their constituent oxides using the Neumann–Kopp rule.
Keywords
- Type
- Invited Paper
- Information
- Journal of Materials Research , Volume 34 , Issue 19: Focus Issue: Thermodynamics of Complex Solids , 14 October 2019 , pp. 3343 - 3350
- Copyright
- Copyright © Materials Research Society 2019
Footnotes
Present address: DECHEMA-Forschungsinstitut, Theodor-Heuss-Allee 25, 60486 Frankfurt am Main, Germany.
This author was an editor of this journal during the review and decision stage. For the JMR policy on review and publication of manuscripts authored by editors, please refer to http://www.mrs.org/editor-manuscripts/.
References
- 6
- Cited by


