Article contents
Enhanced hydrogen generation by solid-state thermal decomposition of NaNH2–NaBH4 composite promoted with Mg–Co–B catalyst
Published online by Cambridge University Press: 06 March 2017
Abstract
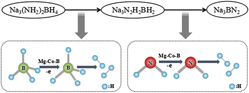
The on-site hydrogen supply is a key issue for the commercialization of the fuel cells, which is one of the important ways for realizing a hydrogen-economy society. Composite NaNH2–NaBH4 is regarded as a promising high-capacity hydrogen storage material. In this paper, the composite NaNH2–NaBH4 (2/1) was synthesized via a solid-state ball milling method. To improve the hydrogen generation kinetics, a multiplex metal boride Mg–Co–B was selected as the catalyst. It was found that Na3BN2 and metal Na were byproducts in the thermal decomposed sample by X-ray diffraction analysis. Thermogravimetry and differential thermal analysis indicated that the main decomposition stages of the catalyst promoted NaNH2–NaBH4 material were split into three stages. The activation energy of the Mg–Co–B promoted NaNH2–NaBH4 (2/1) material below 300 °C was 76.4 KJ/mol, which is only 47.9% of that of the pristine NaNH2–NaBH4 (2/1), and implying much better hydrogen generation kinetics.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2017
Footnotes
Contributing Editor: Xiaobo Chen
References
REFERENCES
- 6
- Cited by



