Article contents
Enhanced grain boundary embrittlement of an Fe grain boundary segregated by hydrogen (H)
Published online by Cambridge University Press: 04 May 2012
Abstract
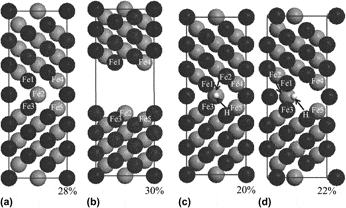
First-principles fully relaxed tensile and shear test simulations were performed on  tilt Fe grain-boundaries (GBs) with and without hydrogen (H) segregation, to investigate the mechanisms of GB embrittlement enhanced by H segregation. Premature fracture was found in the H-segregated GB, compared with the clean GB, in the tensile test simulations. The Fe–H bond showed covalent-like and ion-like characteristics. The covalent-like characteristics reinforced the Fe–Fe bonds, but the ion-like characteristics weakened the Fe–Fe bonds as a result of charge transfer. The effect of the latter increased with increasing strain, and prevailed over the former, resulting in GB embrittlement. In the shear test simulations, variation in the GB energy for the H-segregated GB was almost the same as that for the clean GB. This is because bond-breaking and rebonding occur concurrently in GB shearing and the variations in charge transfer during shear straining are less than those during tensile straining.
tilt Fe grain-boundaries (GBs) with and without hydrogen (H) segregation, to investigate the mechanisms of GB embrittlement enhanced by H segregation. Premature fracture was found in the H-segregated GB, compared with the clean GB, in the tensile test simulations. The Fe–H bond showed covalent-like and ion-like characteristics. The covalent-like characteristics reinforced the Fe–Fe bonds, but the ion-like characteristics weakened the Fe–Fe bonds as a result of charge transfer. The effect of the latter increased with increasing strain, and prevailed over the former, resulting in GB embrittlement. In the shear test simulations, variation in the GB energy for the H-segregated GB was almost the same as that for the clean GB. This is because bond-breaking and rebonding occur concurrently in GB shearing and the variations in charge transfer during shear straining are less than those during tensile straining.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2012
References
REFERENCES
- 11
- Cited by


