Published online by Cambridge University Press: 10 May 2013
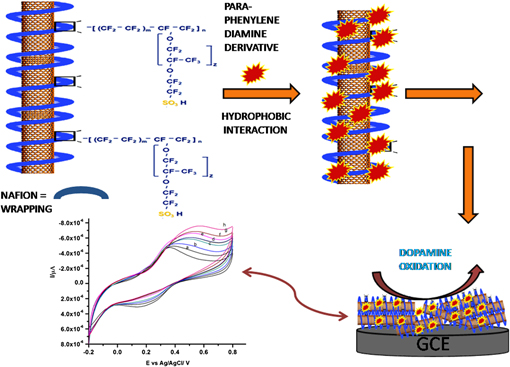
A nanocomposite-modified electrode has been prepared by functionalizing multiwalled carbon nanotubes (MWCNTs) with N-(1,3-dimethylbutyl)-N′-phenyl-p-phenylenediamine (p-PDA). The physical characterization of the prepared composite has been done using infrared spectroscopy and ultraviolet-visible spectroscopy. The morphologies of the prepared p-PDA/MWCNTs/ionophore film have been characterized using scanning electron microscopy and transmission electron microscopy. The electrochemical studies of the prepared composite electrode have been investigated by cyclic voltammetry technique. We observed that the p-PDA/MWCNTs/ionomer composite has better electrochemistry, film adhesion with homogeneous dispersion at the electrode surface and an electrocatalytic activity toward the oxidation of dopamine (DA) in 0.1 M phosphate buffer solution (pH 7.0) at a potential of 50 mV. The linear range and detection limit for the detection of DA was found to be 62–625 and 5 µM respectively. The modified electrode also exhibited several attractive features such as simple preparation, fast response, good stability and repeatability.