Article contents
Electrochemically directed two-component monolayers on gold
Published online by Cambridge University Press: 24 January 2011
Abstract
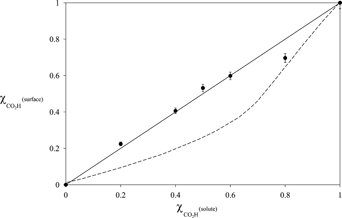
Self-assembled monolayers (SAMs) were formed on gold at anodic potentials from solutions containing two different alkyl thiosulfates, CH3(CH2)10S2O3Na and HO2C(CH2)10S2O3Na. The resulting two-component SAMs were analyzed using x-ray photoelectron spectroscopy to relate their compositions to those of the solutions from which they were adsorbed. This relationship was more linear than reported for analogous SAMs adsorbed from mixed solutions of alkanethiols. The wettability of these surfaces by water and by hexadecane was also measured and compared to analogous SAMs prepared by chemisorption of thiols from solution.
- Type
- Reviews
- Information
- Journal of Materials Research , Volume 26 , Issue 2: Focus Issue: Self-Assembly and Directed Assembly of Advanced Materials , 28 January 2011 , pp. 262 - 267
- Copyright
- Copyright © Materials Research Society 2011
References
REFERENCES
- 2
- Cited by


