Article contents
Effects of high-temperature ambient on cyclic fatigue of La0.8Sr0.2MnO3+δ
Published online by Cambridge University Press: 15 August 2011
Abstract
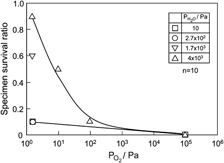
The effects of water vapor and oxygen on the cyclic fatigue behavior of oxygen-excess La0.8Sr0.2MnO3+δ (LSM) were investigated under three-point bending at 1273 K. Because the fatigue life did not obviously depend on the number of cycles, which also represented the effective time of the applied stress, the fracture was presumed to not be significantly controlled by stress-corrosion cracking. Under a low oxygen partial pressure ( ), however, wet exposure inhibited both fatigue fracture and permanent deformation, in which the LSM crystal lattice was distorted and the unit cell free volume was reduced. Under a high
), however, wet exposure inhibited both fatigue fracture and permanent deformation, in which the LSM crystal lattice was distorted and the unit cell free volume was reduced. Under a high  , on the contrary, the crystal symmetry was increased by the wet exposure. The inhibition of fatigue fracture and deformation at both high
, on the contrary, the crystal symmetry was increased by the wet exposure. The inhibition of fatigue fracture and deformation at both high  and low
and low  was probably caused by retardation of lanthanum diffusion through its vacancies.
was probably caused by retardation of lanthanum diffusion through its vacancies.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2011
References
REFERENCES
- 2
- Cited by


