Article contents
Controlled loading of paramagnetic gadolinium oxide nanoplates in PMAO-g-PEG as effective T1-weighted MRI contrast agents
Published online by Cambridge University Press: 07 August 2014
Abstract
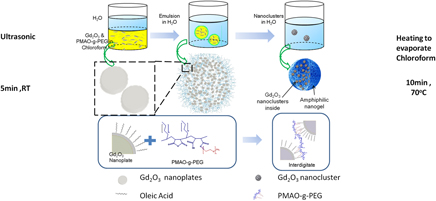
Plate-shaped Gd2O3 nanoclusters (GNCs) with well-controlled loading were fabricated by using amphiphilic poly(maleic anhydride-alt-1-octadecene) (PMAO) grafted with PEG as nanogel. The hydrodynamic size of the obtained GNCs was well controlled to <260 nm under appropriate emulsion process conditions and they showed excellent long-term dispersibility in phosphate buffer saline. MRI measurements clearly indicated the substantial improvement in T1 effect of the nanoclusters as compared with the individual Gd2O3 nanoplates. The obtained GNCs possessed a high r1 value of 7.948 s−1mM−1 [Gd], which is 2.23 times higher than that of the commercial product Gd-DOTA, and low r2/r1 of 1.04. In vitro test of the obtained GNCs was demonstrated in NIH/3T3 cell lines, and clear T1-weighted images were obtained. Thus, the PMAO-g-PEG assisted GNCs were potentially useful for T1-weighted MRI contrast agents.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2014
References
REFERENCES
- 8
- Cited by


