Article contents
The control of morphological and size properties of carbamazepine-imprinted microspheres and nanospheres under different synthesis conditions
Published online by Cambridge University Press: 27 September 2013
Abstract
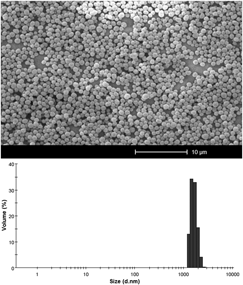
Potential applications of the molecularly imprinted polymers (MIPs) demand physical configurations in different size ranges. Nowadays, research on MIPs is focused on the development of new or improved morphologies, which involves control and modification of the different parameters during the synthesis. In this study, the effect of different synthesis conditions on the particle size and morphology is investigated. Carbamazepine-imprinted polymers were prepared using precipitation polymerization under various conditions such as: the amounts of cross-linker and functional monomers, initiator, porogen, temperature and time of polymerization. We studied the polymerization conditions to obtain imprinted spherical particles with controllable sizes in the range of 243 nm to 3.4 μm. Scanning electron microscopy and photon correlation spectroscopy were utilized to investigate the morphological characterization. The mole ratio of the functional monomer to the cross-linker was important to obtain smaller uniformly sized particles. In addition, the results showed that the cross-linker in the molecular imprinting synthesis can affect the morphology and composition of the MIPs, which influences the binding affinity.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2013
References
REFERENCES
- 10
- Cited by


