I. INTRODUCTION
Graphene, which consists of one atom thick sp2-bonded graphite, received the attention of different disciplines of physics because of its marvelous properties. Reference Novoselov, Geim, Morozov, Jiang, Zhang, Dubonos, Grigorieva and Firsov1–Reference Bonaccorso, Sun, Hasan and Ferrari4 To fully utilize the graphene in industry, fabrication of high quality graphene is essential. The exfoliated single-layer graphene exhibit carrier mobility of ∼10,000 cm2/V s when transferred on Si wafer and 200,000 cm2/V s in its suspended form but the area coverage and number of layers are not well controlled. The most promising method to grow large area graphene on metal catalysts is chemical vapor deposition (CVD). Reference Reina, Jia, Ho, Nezich, Son, Bulovic, Dresselhaus and Kong5–Reference Kim, Zhao, Jang, Lee, Kim, Kim, Ahn, Kim, Choi and Hong7 The problem widely faced by the scientific community in the CVD growth is the presence of crystallographic graphene domains. The growth of graphene is initiated at different nucleation sites on the metal catalyst which give full coverage with the increase in growth time but also promote the growth of few layer grains having different crystallographic orientation. During the growth procedure, nucleation density of graphene plays an important role because it controls grain boundaries which would affect the quality of graphene. The material properties like mechanical strength, mobility, doping percentage, and thermal transport are greatly affected by these grain boundaries. Reference Vlassiouk, Smirnov, Ivanov, Fulvio, Dai, Meyer, Chi, Hensley, Datskos and Lavrik8 The grown single layer graphene (SLG) grown via CVD mostly shows lower mobility ∼3000 cm2/V s as compare to mechanically exfoliated graphene. Reference Song, Li, Miyazaki, Sato, Hayashi, Yamada, Yokoyama and Tsukagosh9 To consistently fabricate high quality graphene, there is still more to be understood about the growth parameters and the nucleation of domains.
Recently, attention has been paid to decrease the nucleation density of graphene by growing single layer crystalline grains of few millimetre in size. Reference Zhou, Yu, Liu, Cheng, Chen, Huang, Liu, Wang, Huang and Duan10 Large area grains were grown on metal catalysts like Cu Reference Zhou, Yu, Liu, Cheng, Chen, Huang, Liu, Wang, Huang and Duan10–Reference Li, Magnuson, Venugopal, Tromp, Hannon, Vogel, Colombo and Ruoff13 and Pt Reference Gao, Ren, Xu, Jin, Wang, Ma, Ma, Zhang, Fu, Peng, Bao and Cheng14 using low and ambient pressure CVD (APCVD) technique, but there is no final recipe which can eliminate the nucleation sites. It is challenging to obtain a continuous layer of graphene without graphene domains using CVD. Effect of different parameters like catalyst annealing time, Reference Wang, Wang, Bao, Yang, Zhu, Xie and Zhang11 precursor gas pressures, Reference Yan, Lin, Peng, Sun, Zhu, Li, Xiang, Samuel, Kittrell and Tour15,Reference Li, Magnuson, Venugopal, An, Suk, Han, Borysiak, Cai, Velamakanni, Zhu, Fu, Vogel, Voelkl, Colombo and Ruoff16 and growth temperature Reference Vlassiouk, Regmi, Fulvio, Dai, Datskos, Eres and Smirnov17 was widely studied for graphene domains grown on Cu using CVD but very few reports have been published for graphene grown on Pt. In materials, the process of nucleation, growth, and coalescence of grains breaks periodicity and exhibit polycrystalline structure. The grain boundaries impede dislocation motion in metals which mechanically strengthen them, but on the other side increases the electron scattering which affect the conductivity. Graphene researchers are concerned about the nucleation and the presence of graphene domains on catalyst surfaces which affect the growth and crystallinity of SLG. If nucleation sites would be located far away on the metal catalyst and the growth would take place quickly, then it is possible to grow large area SLG but growth must be isotropic to reduce defects in graphene. Recently, Gao et al. were successful in growing millimetre size graphene grains on Pt with high crystallinity. Reference Gao, Ren, Xu, Jin, Wang, Ma, Ma, Zhang, Fu, Peng, Bao and Cheng14 A lot of parameters still need to be studied for graphene growth on Pt. In this study, graphene via CVD was grown on Pt and the evolution of graphene grains from smaller to bigger size were observed. Further, a particular set of overlapped grains and their stacking sequences were investigated.
II. EXPERIMENTS
Graphene grains were grown on Pt foils in APCVD system using CH4 (99.995% purity, Messer, Ankara, Turkey), H2 (99.999%, BOS, Ankara, Turkey), and Argon (99.999% purity, Messer) precursor gases. The graphene grains were grown at 1 sccm CH4 flow rate for 3 h at 1040 °C. Initially, the Ar gas flowed at a rate of 300 sccm for 15 min to purge the system and the hydrogen gas was introduced afterward at the rate of 50 sccm. The hydrogen flow was maintained at 150 sccm when the temperature reached 800 °C and was maintained at the end of the experiment. At 1040 °C, CH4 was introduced into the system at a flow rate of 1 sccm for 3 h to grow the graphene. After growth, the furnace was opened to cool down the samples quickly.
The graphene on Pt was coated with C9-PMMA electron beam resist to provide mechanical support during transfer. The graphene was transferred using a hydrogen bubbling technique, Reference Gao, Ren, Xu, Jin, Wang, Ma, Ma, Zhang, Fu, Peng, Bao and Cheng14 the hydrogen bubbles produced during electrolysis of lithium hydroxide reach toward PMMA/graphene/Pt electrode and try to stick with Pt and as a result peel off graphene. The graphene/PMMA stack was scooped out and transferred to deionized (DI) water. After cleaning in water, the stack was transferred to SiO2 covered Si and left overnight in the air for drying. After drying, samples were heated at 180 °C on a hot plate followed by vacuum drying at 70 °C for 3 h. Reference Yan, Lin, Peng, Sun, Zhu, Li, Xiang, Samuel, Kittrell and Tour15 The PMMA layer was dissolved in acetone and the sample was cleaned with methanol, followed by a nitrogen blow dry. Optical microscopes (Carl Zeiss Axio Scope A1 MaT, Jena, Germany, and Nikon Eclipse LV 100, Tokyo, Japan) were used to see the graphene domains and their evolution. The samples were also imaged using Zeiss Supra 35VP field emission scanning electron microscope (Carl Zeiss, Jena, Germany). Renishaw inVia Reflex microRaman spectrometer with 532 nm laser source is used to measure the Raman spectra.
III. RESULTS AND DISCUSSION
During CVD growth, hydrocarbon species react with the presence of metal thin foils due to high temperatures (900–1100 °C). The metal catalyst plays an active role in the decomposition of hydrocarbon and the nucleation of carbon species. Different factors like carbon solubility in the metal catalyst, crystal structure of the catalyst, and the growth conditions play their role in the growth mechanism of graphene. The growth of graphene is due to the solubility of carbon species in the metal, but the solubility is different for different metal catalysts. For Cu, the solubility of carbon is lower (∼0.001 at.%) and the growth of graphene only takes place at the surface of the metal. For Pt, the solubility of carbon is higher (∼0.9%) and the chances of graphene growth would be a combination of two processes i.e., diffusion and precipitation. Reference Bhaviripudi, Jia, Dresselhaus and Kong18 The growth of graphene takes place due to carbon nucleation on Pt surface and the expansion of graphene grains in two dimensions. Graphene has very little interaction with the Pt and can easily expand and cross the grain boundaries of the metal. The stronger affinity of the carbon–carbon atoms helps them to be self assembled in sp2 coordination and expand efficiently on the metal catalyst. Moreover, the weak interactions between the graphene and the Pt substrate causes the formation of rotational domains. Reference Sutter, Sadowski and Sutter19 Theoretically, the mismatch between Moiré superstructure unit cell and Pt atoms causes the rotational domains and they are expected to occur frequently. Reference Merino, Svec, Pinnardi, Otero and Martin-Gago20
Figure 1 shows optical images of different grains which are combining together. Figure 1(a) shows more nucleation sites on the Pt grain boundaries as compared to the plain area. The cause of abundant nucleation at the grain boundaries is the surface roughness of Pt metal which may increase due to repetitive use. Figures 1(b)–1(d) shows different grains combining together and expanding. We grow graphene for 3 h which allows the nucleation and growth of multiple grains and their coalesence with each other. It is well known that in CVD growth method, a second layer of graphene nucleate and grow under the first layer with the same nucleation site. Reference Nie, Walter, Bartelt, Starodub, Bostwick, Rotenberg and McCarty21 A lot of understanding has been gained on the SLG formation where the first layer forms due to supersaturation of carbon gas adatoms. The adatom concentration is very important in growth, most of the carbon islands nucleate at defect sites on the metal catalyst.

FIG. 1. (a) Optical image of individual graphene grains and chain of grains at metal boundaries, (b) optical image of two graphene grains coalesced together, (c) optical image of three graphene grains coalesced together and (d) optical image of the few grains coalesced together.
The fully developed grains show Bernal and twisted stacking, however, the dominance of twisted stacking in fully developed grains were higher.
Figure 2 shows SEM images of single and coalesced graphene grains. The graphene grains were hexagonal in shape before coalescence as seen from the trend of other grains in the sample. The hexagonal grains exhibits corners of 120°, suggesting that their edges are parallel to specific crystallographic directions. It is worthy to note here that the hexagonal shape is different from the flower-like shape of graphite grains, which can be obtained by low pressure CVD. Reference Li, Cai, An, Kim, Nah, Yang, Piner, Velamakanni, Jung, Tutuc, Banerjee, Colombo and Ruoff6,Reference Li, Magnuson, Venugopal, An, Suk, Han, Borysiak, Cai, Velamakanni, Zhu, Fu, Vogel, Voelkl, Colombo and Ruoff16 In APCVD hexagonal graphene grains form with straight edges. The edges of two graphene grains line up parallel during growth and one grain overlaps another. Reference Robertson, Bachmatiuk, Wu, Schäffel, Rellinghaus, Büchner, Rümmeli and Warner22 The overlapping grain maintains the stacking of the two grains. Reference Rao, Pierce and Harutyunyanar23 In case of fewer grains, their edges line up parallel to each other and overlap another. It is desired that if somehow cystallographic orientations of neighboring domains align then it will give a continuous film which will surely decrease the detrimental effect of grain boundaries. In SEM images, the grain boundaries of the coalesced grains are not visible. To be sure about the removal of grain boundaries at the places where graphene grains are combining, we performed Raman mapping of different coalesced grains. Raman contour maps are plotted in Origin and Matlab and images are constructed from the intensities of I G, I D, and I 2D Raman bands.

FIG. 2. (a) SEM image of graphene grain showed in Fig. 1(a), (b) SEM image of two graphene grains coalesced together, (c) SEM image of three graphene grains coalesced together and (d) SEM image of few grains coalesced together.
Raman spectroscopy has been used extensively to investigate the structural and electronic characteristics of graphitic materials. It provides useful information about the defects (D band), the stacking order (2D band) as well as in-plane vibration of sp2 carbon atoms (G band). Reference Reich and Thomsen24–Reference Dresselhaus, Dresselhaus, Saito and Jorio26 The Raman bands of graphene give a clear insight about different phenomena, like, the 2D band simply gives information about whether the graphene is single or multi layer by fitting multiple peaks due to splitting of electronic band structure. Reference Ferrari, Meyer, Scardaci, Casiraghi, Lazzeri, Mauri, Piscanec, Jiang, Novoselov, Roth and Geim27,Reference Cancado, Reina, Kong and Dresselhaus28 The general features of Raman spectrum for graphene lie in the range of 1000–3000 cm−1 with three characteristic bands D (∼1350 cm−1), G (∼1580 cm−1), and 2D (∼2670 cm−1). The D band that originates from transverse optical phonon lies around the K point of the Brillouin zone and activates in the presence of defects. Reference Fang, Hsu, Caudillo, Song, Birdwell, Zakar, Kalbac, Dubey, Palacios, Dresselhaus, Araujo and Kong29 The intensity of the D band gives information about the crystalline quality of a sample. Reference Ferrari30 The G band is a doubly degenerate phonon mode (E 2g symmetry) at the Brillouin zone center and originates due to in-plane vibration of sp2 bonded carbon atoms. The width of the G band gives information about the doping level of a sample. Reference Das, Pisana, Chakraborty, Piscanec, Saha, Waghmare, Novoselov, Krishnamurthy, Geim, Ferrari and Sood31 The 2D or G′ band is the second most prominent band in graphene after D band which arises from the scattering of two equal and opposite phonon wave vectors. In the first Brillouin zone, Raman double resonance phenomena involves two phonons and electronic states which lie near two nonequivalent K points and create a 2D peak in the Raman spectrum. Reference Gayathri, Jayabal, Kottaisamy and Ramakrishnan32,Reference Obraztsova, Osadchy, Obraztsova, Lefrant and Yaminsky33 Moreover, a shift in the position of the 2D band gives information about the strain in the sample. Reference Huang, Yan, Chen, Song, Heinz and Hone34
The electrons exhibit linear dispersion near Dirac K points and behave as massless Dirac fermions in graphene. Reference Novoselov, Geim, Morozov, Katsnelson, Grigorieva, Dubonos and Firsov35,Reference Geim and Novoselov36 In AB stacked bilayer graphene, the valence and conduction band splits into two parabolic nodes near the K point because of the interaction of p electrons and at this point electrons behave as massive Dirac fermions instead of massless Dirac fermions. Reference Geim and Novoselov36,Reference Castro, Novoselov, Morozov, Peres, Lopes dos Santos, Nilsson, Guinea, Geim and Castro Neto37 Interestingly, the band gap of bilayer graphene system can be tuned by applying electric field biasing and the addition of few layers reveals variation in the electronic structure of graphene. Reference Ohta, Bostwick, Seyller, Horn and Rotenberg38 Apart from AB stacking, twisted or arbitrary stacking also exhibits unique properties because of different crystalline structure and its interaction with p electrons.
Figures 3–5 shows Raman spectrum of coalesced grains with their intensity maps. We observed a very small D peak in the samples which show good crystalline quality of the graphene layer. The most promising changes can be seen in the peak positions and full width at half maximum (FWHM) of 2D peak in different regions of coalesced grains which clearly show different number of layers. The change in position and width of 2D peak hints toward different stacking sequences in the coalesced grains which supports the findings of Rao et al. Reference Rao, Pierce and Harutyunyanar23 that two grains, aligned parallel to each other, maintain the stacking sequence. In Fig. 3(a), optical micrograph was marked for different regions which shows single and few layer regions of grains merging together. According to double resonance model, four transitions are involved in the emergence of the 2D peak, i.e., excitation of electron–hole pair via a laser, scattering, backscattering of excited electron by two phonons, and electron hole-pair recombination. Reference Ferrari, Meyer, Scardaci, Casiraghi, Lazzeri, Mauri, Piscanec, Jiang, Novoselov, Roth and Geim27 This process creates two phonons near K and K′ in the Brillouin zone and reflects evolution in the electronic band structure of graphene. For SLG, the 2D peak is symmetric and is fitted with one Lorentz peak which indicates that one Raman cycle is excited near the K and K′ points. Reference Hao, Wang, Wang, Ni, Wang, Wang, Keong Koo, Shen and Thong39 The Raman spectrum of marked region 1 shows SLG with FWHM of 30.18 cm−1 located at 2681.84 cm−1 with an intensity ratio (I 2D/I G) of 1.45. The typical intensity of the 2D peak (I 2D) is more than double of the intensity of G peak (I G) for SLG and it has been observed at region 1. The Raman spectrum of region 2 shows broader FWHM of 55.43 cm−1 located around 2691.81 cm−1 with an intensity ratio (I 2D/I G) of 1.01. From the contrast of the optical micrograph, region 2 appears to be bilayer graphene but carefully examining the intensity ratio map shows that it is actually a trilayer graphene which is supported by the intensity ratio value, which is less than that of SLG. The FWHM for trilayer graphene appeared around 56.2 ± 1.6 cm−1 (Ref. Reference Hao, Wang, Wang, Ni, Wang, Wang, Keong Koo, Shen and Thong39) and matches well with the region 2 FWHM of our samples.

FIG. 3. (a) Marked optical image of graphene grain for Raman spectrum at different points, (b) Raman spectra for Bernal (AB) stack coalesced grains at regions 1, 2, 3, 4, and 5, (c) 2D peak for all the regions, (d) intensity maps for D, G and 2D peaks plotted in matlab and (e) I 2D/I G ratio contour mapping in Origin 8.5 software.

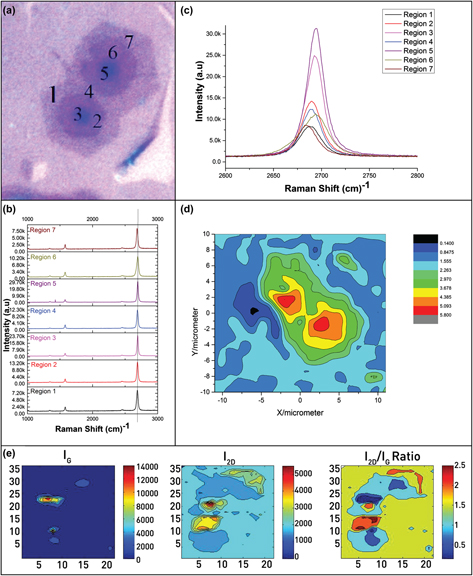
FIG. 4. (a) Marked optical image of graphene grains for Raman spectrum at different points, (b) Raman spectra for twisted stack coalesced grains at regions 1, 2, 3, 4, 5, 6, and 7, (c) 2D peak for all the regions, (d) I 2D/I G ratio contour mapping in Origin 8.5 software and (e) intensity maps for G and 2D peaks plotted in matlab.

FIG. 5. (a) Marked optical image of graphene grains for Raman spectrum at different points, (b) Raman spectra for Bernal (AB) stack coalesced grains at regions 1, 2, 3, 4, and 5, (c) 2D peak for all the regions, (d) intensity maps for D, G, and 2D peaks plotted in matlab and (e) I 2D/I G ratio contour mapping in Origin 8.5 software.
For AB stacked bilayer system, the π electrons split the valence and conduction bands into four parabolic bands (π1, π2, π1*, and π2*) near the K point, Reference Malard, Nilsson, Elias, Brant, Plentz, Alves, Castro Neto and Pimenta40 and two of them have higher relative intensities than the other two. Reference Ferrari, Meyer, Scardaci, Casiraghi, Lazzeri, Mauri, Piscanec, Jiang, Novoselov, Roth and Geim27 The 2D peak shows dispersive behavior for bi-layer graphene and it would be fitted with four Lorentzian peaks with slightly different frequencies and the FWHM is almost twice than that of SLG. With the increase in number of graphene layers, there will be a significant decrease in the intensity of 2D peak components, the electronic bands split into complex configuration and the excited electron–hole pair undergoes more scattering cycles as compared to bilayer graphene and it causes dispersion or widening in the Raman spectrum of the 2D peak for tri or tetra layers. Reference Hao, Wang, Wang, Ni, Wang, Wang, Keong Koo, Shen and Thong39,Reference Malard, Nilsson, Elias, Brant, Plentz, Alves, Castro Neto and Pimenta40 The Raman spectrum for region 3 shows FWHM of 42.72 cm−1 observed around 2694.28 cm−1 with an intensity ratio (I 2D/I G) of 0.28. Optical micrograph, intensity ratio value and consistent increase in peak position hints toward tetra layer AB stacked graphene but the decrease in FWHM value hints toward the folding of graphene. Reference Hao, Wang, Wang, Ni, Wang, Wang, Keong Koo, Shen and Thong39 The Raman spectrum for region 4 shows broader FWHM of 64.52 cm−1 observed around 2700.85 cm−1 with an intensity ratio (I 2D/I G) of 0.23 and predict tetra layer graphene. Reference Hao, Wang, Wang, Ni, Wang, Wang, Keong Koo, Shen and Thong39 The Raman spectrum for region 5 showed FWHM of 52.91 cm−1 observed around 2690.02 cm−1 with an intensity ratio (I 2D/I G) of 0.46 hints bilayer graphene. Reference Hao, Wang, Wang, Ni, Wang, Wang, Keong Koo, Shen and Thong39 Liu et al. Reference Liu, Zhou, Cheng, Yu, Liu, Chen, Shaw, Zhong, Huang and Duan41 observed FWHM values of the 2D peak in the range of 47.5–62.0 cm−1 for the AB stacked bilayer graphene having I 2D/I G ratios in the range of 0.83–1.46. Carefully analysing the contouring boundaries of the coalesced grains in Fig. 3(d), the values of FWHM for different graphene layers match with the predicted values of Hao et al. Reference Hao, Wang, Wang, Ni, Wang, Wang, Keong Koo, Shen and Thong39
Figure 4 shows the Raman spectra for two twisted grains combine adjacently. In Fig. 4(a), optical micrograph was marked for different regions of grains. The twisted or misaligned layers significantly affects the mechanical, Reference Zhang and Zhao42 thermal, Reference Cocemasov, Nika and Balandin43 electrical, Reference Luican, Li, Reina, Kong, Nair, Novoselov, Geim and Andrei44 and optical Reference Wang, Ni, Liu, Liu, Cong, Yu, Wang, Shen and Shen45 properties of graphene. The 2D Raman peak shows a complicated behavior for twisted layer graphene. Theoretically, the twisted layers exhibit linear dispersion near the Dirac point with low energy von-Hove singularities. The electronic structure of twisted layers graphene looks similar to single layer but with lesser Fermi velocity, which means, smaller slope of electronic band near K points. Reference Beechem, Ohta, Diaconescu and Robinson46 The orientation angle is very important in twisted layers because it evolves additional symmetry in the form of Moiré superlattice. Reference Beechem, Ohta, Diaconescu and Robinson46,Reference Lopes dos Santos, Peres and Castro Neto47 Moiré superlattice spreads an extra periodicity which imparts a periodic potential and this periodic potential caused changes in the band structure of twisted layers. Reference Ohta, Robinson, Feibelman, Bostwick, Rotenberg and Beechem48,Reference Beechem, Ohta, Diaconescu and Robinson46 The change in layer orientation is natural in CVD and causes dispersion in band structure which changes the properties of twisted layers from other stacking. In fact, 2D layered materials have a natural tendency of rotational disorder because van der Waals forces are weak and cannot lock the layers strongly in a particular atomic fashion. So, the individual atomic planes have tendency to slide and rotate with respect to each other.
The 2D Raman peak for regions 1, 2, and 3 appeared around 2687.96, 2689.70, and 2692.83 cm−1 with FWHM values of 25.29, 22.34, and 20.23 cm−1, respectively. There is a consistent decrease in FWHM values of the layers with the increase in number. In comparison with SLG (30.18 cm−1), there is a decrease of 4.89 cm−1 (bilayer), 7.84 cm−1 (trilayer), and 9.95 cm−1 (few layer) in the values of FWHM. The I 2D/I G ratio for regions 1, 2, and 3 is 3.42, 4.33, and 5.81, respectively, which is quiet higher than the values of Bernal stacked graphene layers shown in Fig. 3. The higher intensity ratios support the twisted sequence in layers. We observed similar finding in the adjacent grain marked as regions 4, 5, 6, and 7. The common feature of all the regions is the shape of 2D peak which is symmetrical as compare to Bernal stacked grain. Intensity maps clearly showed different number of layers.
Figure 5 shows a complicated grain structure where three grains coalescing together. The 2D Raman peak for regions 1, 3, 4, and 5 appeared around 2696.76, 2695.20, 2693.83, and 2699.42 cm−1 with FWHM values of 57.23 cm−1 (trilayer), 61.08 cm−1 (tetralayer), 56.36 cm−1 (trilayer), and 69.39 cm−1 (pentalayer), respectively. Reference Hao, Wang, Wang, Ni, Wang, Wang, Keong Koo, Shen and Thong39 Moreover, the values of I 2D/I G ratio for regions 1, 3, 4, and 5 is 1.00, 0.81, 0.96, and 0.64, respectively which clearly showed Bernal stacking in the grain. Interestingly, the 2D Raman peak of region 2 shows FWHM value of 20.53 cm−1. The shape of the peak is quite symmetric as compare to other regions and showed I 2D/I G ratio of 4.69 hint toward twisted or disoriented stacking of layers. Reference Liu, Zhou, Cheng, Yu, Liu, Chen, Shaw, Zhong, Huang and Duan41 We believe this region is the area where the grain boundary of the grains are combining which caused change in the orientation of the layers.
IV. CONCLUSION
The present work is about the synthesis of few layer graphene grains on Pt foil using CVD. The graphene grains were analyzed in the coalesced condition. Grains of different stacking sequences were observed in the samples, the occurrence of rotational domains are seen to be higher as compare to Bernal domains due to mismatch between Moiré superstructure unit cell and Pt atoms. A systematic characterization was done to understand the stacking sequence in different grains. The preliminary understanding was obtained using optical microscopy. SEM characterization was done to see the magnified form of coalesced grains. Raman spectroscopy clearly showed stacking sequences in the grains when they are merging with each and growing in bigger size. 2D Raman peak of three different coalesced grains were characterized which showed Bernal AB and twisted layer stacking in the grains. Raman contour mapping also provide imaging of the number of layers which is helpful to get an idea about the number of layers.
ACKNOWLEDGMENTS
S. Karamat would like to thank TÜBİTAK and European Union Marie-Curie Co-Funded 2236 Fellowship for accomplishing this work.