Published online by Cambridge University Press: 05 January 2017
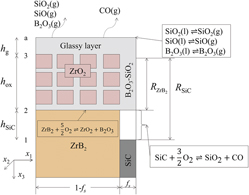
A chemomechanical coupling model is presented in the temperature range of 1200–1800 °C based on the microstructure during oxidation of ZrB2–SiC. The model includes the interaction of the oxidation rate and the mechanical stress. The stress is generated due to the constraint from the substrate to the lateral growth. The generated stress results in the shrink of the pores in the oxide. At the outer glassy layer surface, the boundary layer evaporation is adopted to describe the evaporation rate. Using the coupling model, the evolutions of the oxide layer thickness, weight gain, pore radius, and stress in both the oxide and substrate are provided, and the theoretical calculated results agree well with the reported experimental results. The results reveal large stress in the oxide layer during the oxidation process. By comparing the results of ZrB2 with different volume fractions of SiC, it is found that ZrB2 with higher volume fraction of SiC has more excellent oxidation resistance and smaller stress.
Contributing Editor: Yanchun Zhou