Article contents
Characteristics of direct-patternable SnO2:Pt nanocomposite thin films fabricated by photochemical metal-organic deposition
Published online by Cambridge University Press: 17 October 2011
Abstract
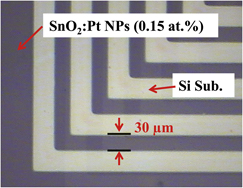
We evaluated the optical and electrical characteristics of SnO2 hybrid films with various contents (0, 0.05, 0.1, 0.15, and 0.2 at.%) of Pt nanoparticles. The Pt nanoparticles were synthesized by a methanol reduction method, and their size was restricted to an average of 3 nm using poly(N-vinyl-2-pyrrolidone) as a protecting agent. An enhancement in electrical properties was observed due to the addition of Pt nanoparticles; the lowest resistivity (1.36 × 10−2 Ω·cm) and the highest figure of merit (1.18 × 10−4 Ω−1) were obtained with SnO2 film containing 0.15 at.% Pt nanoparticles after annealing at 600 °C, and the average transmittance in the visible region was 86.61%. Well-defined 30-μm-wide direct-patterned SnO2 films containing Pt nanoparticles were formed by photochemical metal-organic deposition through a simple process including a photosensitive starting precursor, ultraviolet exposure, and removal of the unexposed area with solvent rinsing.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2011
References
REFERENCES
- 1
- Cited by


