Article contents
Biocomposites based on collagen and phosphorylated dextran for bone regeneration
Published online by Cambridge University Press: 14 March 2012
Abstract
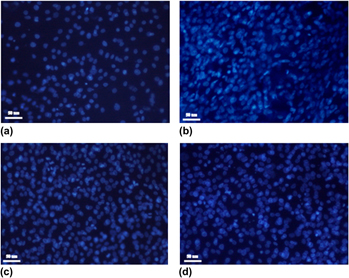
The aim of this study was the development of biocomposite scaffolds (membranes and matrices) based on natural polymers used for bone tissue engineering. The novelty featured in this paper is the use of phosphorylated dextran (PDex) as natural component in collagen-based biocomposites. The PDex both in acid form and as mixed salts of Mg–Na, Zn–Na, Ca-Na was characterized by Fourier transform infrared spectroscopy (FTIR), nuclear magnetic resonance (NMR) spectroscopy, potentiometric and conductometric titration and energy dispersive x-ray spectroscopy (EDX) analysis. The biocomposite scaffolds were obtained by freeze-drying as matrices and by free-drying as membranes with specific microporous morphological structures that depended on drying process of collagen gels with PDex. The biocomposites were physical–chemical characterized by differential scanning calorimetry (DSC) and, water and water vapor absorption. The biocompatibility was evaluated in vitro with human osteosarcoma MG 63 cell lines. The results showed that biocompatibility was improved by the use of PDex as mixed salts of Mg–Na, Zn–Na, Ca-Na in collagen biocomposites.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2012
References
REFERENCES
- 4
- Cited by


