Published online by Cambridge University Press: 31 July 2012
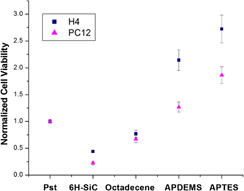
In this article, the biofunctionalization of 6H–SiC (0001) surfaces via self-assembled monolayers (SAMs) has been studied as a means to modify the in vitro biocompatibility of this semiconductor substrate with H4 (human neuroglioma) and PC12 (rat pheochromocytoma) cells. Silanization with aminopropyldiethoxymethylsilane (APDEMS) and aminopropyltriethoxysilane (APTES), which provided moderately hydrophilic surfaces, and alkylation with 1-octadecene that produced hydrophobic surfaces were used to control the 6H–SiC surface chemistry and evaluate changes in cell viability and morphology due to these surface modifications. The morphology of the cells was evaluated with atomic force microscopy. In addition, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays were used to quantitatively evaluate the cell viability on the SAM-modified surfaces. In all cases, the cell proliferation was observed to improve with respect to untreated 6H–SiC surfaces, with up to a 2x increase in viability on the 1-octadecene-modified surfaces, up to 6x increase with APDEMS-modified surfaces, and up to 8x increase with APTES-modified surfaces. This proves the potential of SiC as a substrate for medical devices given the possibility to tailor its surface chemistry for specific applications.